Protein-responsive gut hormone tachykinin directs food choice and impacts lifespan
- PMID: 40229448
- PMCID: PMC12198013
- DOI: 10.1038/s42255-025-01267-0
Protein-responsive gut hormone tachykinin directs food choice and impacts lifespan
Abstract
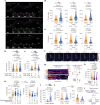
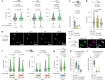
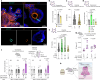
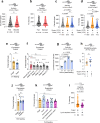
Animals select food based on hungers that reflect dynamic macronutrient needs, but the hormonal mechanisms underlying nutrient-specific appetite regulation remain poorly defined. Here, we identify tachykinin (Tk) as a protein-responsive gut hormone in Drosophila and female mice, regulated by conserved environmental and nutrient-sensing mechanisms. Protein intake activates Tk-expressing enteroendocrine cells (EECs), driving the release of gut Tk through mechanisms involving target of rapamycin (TOR) and transient receptor potential A1 (TrpA1). In flies, we delineate a pathway by which gut Tk controls selective appetite and sleep after protein ingestion, mediated by glucagon-like adipokinetic hormone (AKH) signalling to neurons and adipose tissue. This mechanism suppresses protein appetite, promotes sugar hunger and modulates wakefulness to align behaviour with nutritional needs. Inhibiting protein-responsive gut Tk prolongs lifespan through AKH, revealing a role for nutrient-dependent gut hormone signalling in longevity. Our results provide a framework for understanding EEC-derived nutrient-specific satiety signals and the role of gut hormones in regulating food choice, sleep and lifespan.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- Small, C. J. & Bloom, S. R. Gut hormones and the control of appetite. Trends Endocrinol. Metab.15, 259–263 (2004). - PubMed
-
- Murphy, K. G. & Bloom, S. R. Gut hormones in the control of appetite. Exp. Physiol.89, 507–516 (2004). - PubMed
-
- Munch, D., Ezra-Nevo, G., Francisco, A. P., Tastekin, I. & Ribeiro, C. Nutrient homeostasis — translating internal states to behavior. Curr. Opin. Neurobiol.60, 67–75 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

