Lipin1-dependent transcriptional inactivation of SREBPs contributes to selinexor sensitivity in multiple myeloma
- PMID: 40229499
- PMCID: PMC12373733
- DOI: 10.1038/s41401-025-01553-3
Lipin1-dependent transcriptional inactivation of SREBPs contributes to selinexor sensitivity in multiple myeloma
Abstract
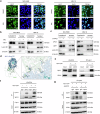
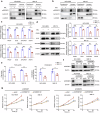
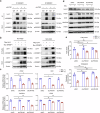
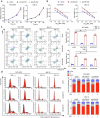
Selective nuclear export inhibitor selinexor (SEL) represents a promising therapeutic strategy for relapsed/refractory multiple myeloma (RRMM). But its mechanisms of action as well as factors that influence therapeutic responses have not been fully characterized yet. In this study we employed catTFRE proteomics technique to profile changes in nuclear abundance of activated transcription factors (TFs)/co-factors (TCs) in myeloma cells following SEL treatment. We found that pharmacological inhibition of exportin-1 (XPO1) by SEL leads to a significant nuclear accumulation of Lipin1 in NCI-H929 cells. Nuclear-localized Lipin1 acted as a transcriptional cofactor that suppressed the transcriptional activity of SREBPs. By performing subcellular localization analysis, molecular docking, co-immunoprecipitation and other assays, we demonstrated that Lipin1 was subjected to XPO1-dependent nuclear export. We demonstrated that SEL downregulated the expression of key lipogenesis-related genes regulated by SREBPs including FASN, SCD, DHCR24 and FDPS, leading to reduced fatty acid and cholesterol synthesis in MM cell lines and primary CD138+ cells. Using shRNA-mediated knockdown assays, we elucidated the critical role of Lipin1 in mediating the inhibitory effects of SEL on the SREBPs pathway and its contribution to SEL sensitivity both in vitro and in murine xenograft models. In conclusion, we reveal a novel mechanism by which SEL downregulates cellular lipid biosynthesis, thereby inhibiting the proliferation of myeloma cells. This study highlights the critical role of Lipin1 in the anti-myeloma effects of SEL, suggesting its potential as a biomarker for identifying patients who are most likely to benefit from SEL-based therapies.
Keywords: Lipin1; SREBPs; exportin-1; multiple myeloma; proteomics.; selinexor.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Kastritis E, Terpos E, Dimopoulos MA. How I treat relapsed multiple myeloma. Blood. 2022;139:2904–17. - PubMed
-
- Richard S, Jagannath S. Targeting nuclear export proteins in multiple myeloma therapy. BioDrugs. 2022;36:13–25. - PubMed
-
- Benkova K, Mihalyova J, Hajek R, Jelinek T. Selinexor, selective inhibitor of nuclear export: unselective bullet for blood cancers. Blood Rev. 2021;46:100758. - PubMed
-
- Delimpasi S, Mateos MV, Auner HW, Gavriatopoulou M, Dimopoulos MA, Quach H, et al. Efficacy and tolerability of once-weekly selinexor, bortezomib, and dexamethasone in comparison with standard twice-weekly bortezomib and dexamethasone in previously treated multiple myeloma with renal impairment: subgroup analysis from the BOSTON study. Am J Hematol. 2022;97:e83–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

