Mesobuthus eupeus venom modulates colorectal carcinoma signaling pathways and induces apoptosis
- PMID: 40229568
- PMCID: PMC11996983
- DOI: 10.1007/s12032-025-02689-2
Mesobuthus eupeus venom modulates colorectal carcinoma signaling pathways and induces apoptosis
Abstract
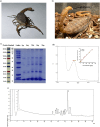
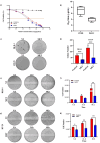
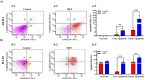
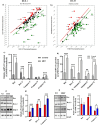
Colorectal cancer (CRC) is a significant global health concern, often challenging to treat effectively with conventional methods and burdened by adverse effects. Scorpion venoms offer a unique avenue for exploration, given their ability to disrupt the cell cycle, inhibit growth, and trigger apoptosis. This study delves into the impact of Mesobuthus eupeus (M. eupeus) scorpion venom on the proliferation and progression of colorectal cancer at the molecular level. The total protein concentration in the venom (607.5 µg/mL) also emphasized the rich composition and potential for therapeutic applications. The study reveals that M. eupeus venom effectively reduced the proliferation of DLD-1 and HT-29 colorectal cancer cells in a dose-dependent manner with IC50 values of 4.32 and 7.61 µg/mL, respectively. The venom also impedes cell migration, diminishes colony formation, and triggers apoptosis in the cancer cells. The venom also induced early and late apoptosis in the two cancer cell lines. The human colorectal cancer and apoptotic pathways were clarified at the molecular level using pathway panels, which revealed that 16 genes involved in colorectal cancer increased while 23 decreased. In the HT-29 cell line, 57 genes increased, and 1 decreased following venom treatment. Besides, the mRNA expression of 19 genes involved in the apoptotic pathway was increased, while 22 were reduced in DLD-1 cells. This study underscores the potential of M. eupeus venom as a natural therapeutic approach in the quest for cancer treatments.
Keywords: Mesobuthus eupeus; Colorectal cancer; Cytotoxicity; Molecular mechanism; Venom.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no competing interests.
Figures
Similar articles
-
F1 fraction isolated from Mesobuthus eupeus scorpion venom induces macrophage polarization toward M1 phenotype and exerts anti-tumoral effects on the CT26 tumor cell line.Int Immunopharmacol. 2024 May 10;132:111960. doi: 10.1016/j.intimp.2024.111960. Epub 2024 Mar 29. Int Immunopharmacol. 2024. PMID: 38554440
-
The neutralizing capacity of Androctonus crassicauda antivenom against Mesobuthus eupeus scorpion venom.Toxicon. 2008 Aug 1;52(2):375-9. doi: 10.1016/j.toxicon.2008.06.019. Epub 2008 Jun 26. Toxicon. 2008. PMID: 18625264
-
Scorpion Venom Causes Apoptosis by Increasing Reactive Oxygen Species and Cell Cycle Arrest in MDA-MB-231 and HCT-8 Cancer Cell Lines.J Evid Based Integr Med. 2018 Jan-Dec;23:2156587217751796. doi: 10.1177/2156587217751796. J Evid Based Integr Med. 2018. PMID: 29405760 Free PMC article.
-
Scorpion Venom Peptides as a Potential Source for Human Drug Candidates.Protein Pept Lett. 2018;25(7):702-708. doi: 10.2174/0929866525666180614114307. Protein Pept Lett. 2018. PMID: 29921194 Review.
-
Pleiotropic Anticancer Properties of Scorpion Venom Peptides: Rhopalurus princeps Venom as an Anticancer Agent.Drug Des Devel Ther. 2020 Feb 27;14:881-893. doi: 10.2147/DDDT.S231008. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32161447 Free PMC article. Review.
References
-
- Piña-Sánchez P, Chávez-González A, Ruiz-Tachiquín M, Vadillo E, Monroy-García A, Montesinos JJ, Grajales R, Gutiérrez de la Barrera M, Mayani H. Cancer biology, epidemiology, and treatment in the 21st century: current status and future challenges from a biomedical perspective. Cancer Control. 2021;28:10732748211038736. 10.1177/10732748211038735. - PMC - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–49. - PubMed
-
- Ansari B, Aschner M, Hussain Y, Efferth T, Khan H. Suppression of colorectal carcinogenesis by naringin. Phytomedicine. 2022;96: 153897. 10.1016/j.phymed.2021.153897. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

