Exploring endothelial dysfunction in major rheumatic diseases: current trends and future directions
- PMID: 40229608
- PMCID: PMC12141126
- DOI: 10.1007/s00109-025-02539-8
Exploring endothelial dysfunction in major rheumatic diseases: current trends and future directions
Abstract
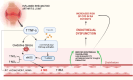
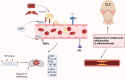
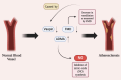
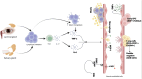
The relationship between rheumatic diseases (RDs) and endothelial dysfunction (ED) is intricate and multifaceted, with chronic inflammation and immune system dysregulation playing key roles. RDs, including Osteoarthritis (OA), Rheumatoid arthritis (RA), Systemic Lupus erythematosus (SLE), Ankylosing spondylitis (AS), Psoriatic arthritis (PsA), Sjogren's syndrome (SS), Systemic sclerosis (SSc), Polymyalgia rheumatica (PMR) are characterized by chronic inflammation and immune dysregulation, leading to ED. ED is marked by reduced nitric oxide (NO) production, increased oxidative stress, and heightened pro-inflammatory and prothrombotic activities, which are crucial in the development of cardiovascular disease (CVD) and systemic inflammation. This association persists even in RD patients without conventional cardiovascular risk factors, suggesting a direct impact of RD-related inflammation on endothelial function. Studies also show that ED significantly contributes to atherosclerosis, thereby elevating cardiovascular risk in RD patients. This review synthesizes the molecular mechanisms connecting major RDs and ED, highlighting potential biomarkers and therapeutic targets. Ultimately, the review aims to enhance understanding of the complex interactions leading to ED in rheumatic patients and inform strategies to mitigate cardiovascular risks and improve patient outcomes.
Keywords: Cardiovascular diseases; Chronic inflammation; Endothelial dysfunction; Immune system dysregulation; Rheumatic diseases.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study does not involve any human or animal testing. Competing interests: The authors declare no competing interests.
Figures

References
-
- Calle E, Gómez-Puerta JA (2018) Chapter 1 - The spectrum of rheumatic diseases. In: Atzeni F et al (eds) Handbook of systemic autoimmune diseases, vol 15. Elsevier, pp 1–13
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

