Erythrocyte-derived extracellular vesicles transcytose across the blood-brain barrier to induce Parkinson's disease-like neurodegeneration
- PMID: 40229767
- PMCID: PMC11998243
- DOI: 10.1186/s12987-025-00646-9
Erythrocyte-derived extracellular vesicles transcytose across the blood-brain barrier to induce Parkinson's disease-like neurodegeneration
Abstract
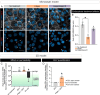
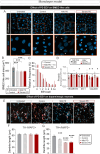
Parkinson's disease (PD) is a neurodegenerative illness characterized by motor and non-motor features. Hallmarks of the disease include an extensive loss of dopaminergic neurons in the substantia nigra pars compacta, evidence of neuroinflammation, and the accumulation of misfolded proteins leading to the formation of Lewy bodies. While PD etiology is complex and identifying a single disease trigger has been a challenge, accumulating evidence indicates that non-neuronal and peripheral factors may likely contribute to disease onset and progression. The brain is shielded from peripheral factors by the blood-brain barrier (BBB), which tightly controls the entry of systemic molecules and cells from the blood to the brain. The BBB integrates molecular signals originating from the luminal (blood) and abluminal (brain) sides of the endothelial wall, regulating these exchanges. Of particular interest are erythrocytes, which are not only the most abundant cell type in the blood, but they also secrete extracellular vesicles (EVs) that display disease-specific signatures over the course of PD. Erythrocyte-derived EVs (EEVs) could provide a route by which pathological molecular signals travel from the periphery to the central nervous system. The primary objective of this study was to evaluate, in a human-based platform, mechanisms of EEV transport from the blood to the brain under physiological conditions. The secondary objective was to determine the ability of EEVs, generated by erythrocytes of healthy donors or patients, to induce PD-like features. We leveraged two in vitro models of the BBB, the transwell chambers and a microfluidic BBB chip generated using human induced pluripotent stem cells. Our findings suggest that EEVs transcytose from the vascular to the brain compartment of the human BBB model via a caveolin-dependant mechanism. Furthermore, EEVs derived from individuals with PD altered BBB integrity compared to healthy EEV controls, and clinical severity aggravated the loss of barrier integrity and increased EEV extravasation into the brain compartment. PD-derived EEVs reduced ZO-1 and Claudin 5 tight junction levels in BMEC-like cells and induced the selective atrophy of dopaminergic neurons. In contrast, non-dopaminergic neurons were not affected by treatment with PD EEVs. In summary, our data suggest that EEV interactions at the human BBB can be studied using a highly translational human-based brain chip model, and EEV toxicity at the neurovascular unit is exacerbated by disease severity.
Keywords: 3D model; Blood brain barrier; Erythrocytes; Extracellular vesicles; Parkinson’s disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Institutional review boards approved this study (CHU de Québec, #A13-2-1096; CHUM, #14.228; Cambridge Central Regional Ethics Committee, REC #03/303 & #08/H0306/26; and Cambridge University Hospitals Foundation Trust Research and Development department, R&D #A085170 & #A091246) in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants. Consent to publish: Consent to Publish declaration: not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- de Rus Jacquet A, Alpaugh M, Denis HL, Tancredi JL, Boutin M, Decaestecker J, Beauparlant C, Herrmann L, Saint-Pierre M, Parent M, Droit A, Breton S, Cicchetti F. The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat Commun. 2023;14:3651. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

