Development of Cre-dependent retrograde trans-multisynaptic tracer based on pseudorabies virus bartha strain
- PMID: 40229811
- PMCID: PMC11995500
- DOI: 10.1186/s13041-025-01204-y
Development of Cre-dependent retrograde trans-multisynaptic tracer based on pseudorabies virus bartha strain
Abstract
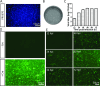
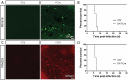
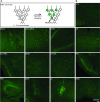
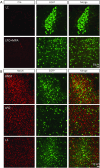
Mapping the neural circuit of a specific neuronal subclass is central to understanding the working mechanism of the brain. Currently, numerous types of transgenic mice expressing Cre recombinase have been engineered and widely used in neuroscience. To map the multilevel inputs into the neural circuit of a specific neuronal subpopulation, a Cre-dependent retrograde trans-multisynaptic tracer must be developed. The vaccine strain of Pseudorabies virus (PRV, Bartha strain) can infect neurons and spread in a retrograde manner in the neural circuit. In this study, we engineered the genome of PRV Bartha strain to prepare two new tracers, PRV676 and PRV829, by replacing the TK gene of PRV with the Cre-dependent expression cassette of the fluorescent protein gene and the TK gene. These two tracers can separately and Cre-dependently express EGFP and mRuby3 and produce progeny viruses in vitro and in vivo, which can help to map the multilevel inputs of a specific neuronal subpopulation expressing Cre. Collectively, our work provides two new tools for neuroscience research.
Keywords: Cre-dependent; PRV676; PRV829; Pseudorabies virus; Retrograde trans-multisynaptic tracer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures were approved by the Animal Care and Use Committees at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Yang XW, Gong S. An overview on the generation of BAC transgenic mice for neuroscience research. Curr Protoc Neurosci 2005, Chap. 5:Unit 5 20. - PubMed
MeSH terms
Substances
Grants and funding
- ZDSYS20200811142401005/Shenzhen Key Laboratory of Viral Vectors for Biomedicine
- 2020ZDB26/Key Laboratory of Quality Control Technology for Virus-Based Therapeutics, Guangdong Provincial Medical Products Administration, Shenzhen
- 2021ZD0201003/STI2030-Major Projects
- 2022KSYS012/Guangdong Provincial Key Laboratory of Viral Biotechnology and Application
- 32071038/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

