Efficacy and safety of dexamethasone versus intravitreal aflibercept implants for macular edema: a systematic review and meta-analysis
- PMID: 40229845
- PMCID: PMC11998416
- DOI: 10.1186/s40001-025-02404-x
Efficacy and safety of dexamethasone versus intravitreal aflibercept implants for macular edema: a systematic review and meta-analysis
Abstract
Background: Macular edema (ME) is a prevalent complication of diabetic retinopathy (DR) and retinal vein occlusion (RVO) that contributes significantly to vision impairment worldwide. This condition is primarily driven by elevated vascular endothelial growth factor (VEGF) and pro-inflammatory cytokines, resulting in the use of anti-VEGF agents such as aflibercept and corticosteroids such as dexamethasone implants. However, evidence comparing the clinical efficacy and safety of these two modalities remains limited.
Objectives: This systematic review and meta-analysis aimed to compare the safety and efficacy of intravitreal aflibercept injections and dexamethasone implants in ME associated with DR and RVO.
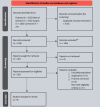
Method: The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered with PROSPERO (CRD42024577212). A comprehensive search of the PubMed, Cochrane, Web of Science, and Scopus databases was performed until August 30, 2024. Nine studies, involving 572 eyes, were included in the analysis. Key outcomes assessed included Best-Corrected Visual Acuity (BCVA), Central Retinal Thickness (CRT), and Intraocular Pressure (IOP). A random-effects model was applied to the pooled effect size calculations, and heterogeneity was addressed using sensitivity analyses.
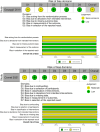
Results: Both treatments showed comparable efficacy in improving BCVA and reducing CRT across follow-up intervals. At 3 months, dexamethasone implants demonstrated statistically significant superiority in BCVA improvement (MD = 1.18, 95% CI [0.89, 1.47], P < 0.001) and CRT reduction (MD = - 62.45 µm, 95% CI [- 85.67, - 39.22], P < 0.001) compared to aflibercept. Similarly, at 12 months, dexamethasone implants maintained greater efficacy in CRT reduction (MD = - 58.73 µm, 95% CI [- 78.12, - 39.34], P < 0.001). However, dexamethasone implants were associated with an increased IOP at 3 and 6 months (MD = 1.04 mmHg, 95% CI [0.56, 1.52], P < 0.001). No significant differences in IOP were observed between treatments at 12 months.
Conclusion: Intravitreal aflibercept injections and dexamethasone implants are effective modalities for the management of ME, with each presenting distinct advantages. Dexamethasone implants minimize the frequency of treatment, while achieving superior outcomes in terms of BCVA and CRT. However, they are also associated with a heightened risk of IOP elevation and cataract formation. Conversely, aflibercept requires more frequent administration, which may result in logistical and financial challenges for patients and health care providers. Therefore, personalized treatment strategies should consider disease severity, comorbidities, and individual preferences. Future research should prioritize patient-centered outcomes, emphasizing quality of life and treatment costs while also investigating condition-specific responses to these therapeutic interventions.
Keywords: Anti-VEGF therapy; Central retinal thickness; Corticosteroids; Dexamethasone implants; Diabetic macular edema (DME); Diabetic retinopathy (DR); Intraocular pressure; Intravitreal aflibercept; Macular edema; Retinal vein occlusion (RVO); Visual acuity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Qiu X-Y, Hu X-F, Qin Y-Z, Ma J-X, Liu Q-P, Qin L, et al. Comparison of intravitreal aflibercept and dexamethasone implant in the treatment of macular edema associated with diabetic retinopathy or retinal vein occlusion: a Meta-analysis and systematic review. Int J Ophthalmol. 2022;15(9):1511–9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

