HD6277 Suppresses Muscle Atrophy by Promoting Myogenic Factors and Inhibiting Proteolysis in Aged Mice
- PMID: 40229990
- PMCID: PMC11996700
- DOI: 10.1002/jcsm.13805
HD6277 Suppresses Muscle Atrophy by Promoting Myogenic Factors and Inhibiting Proteolysis in Aged Mice
Abstract
Background: G protein-coupled receptor 40 (GPR40) acts as a modulator of various physiological functions, including glycaemic lowering, anti-inflammation and antioxidative stress, in several tissues. However, the role of GPR40 in skeletal muscles remains unclear.
Methods: To investigate the roles of muscle GPR40, C2C12 myoblasts and myotubes were stimulated with palmitate and HD6277, a GPR40 agonist. Muscle strength and myofiber thickness were measured in obese and aged mice fed HD6277.
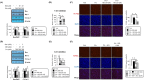
Results: In C2C12 myoblasts, the addition of HD6277 induced phosphorylated Akt levels and expression of the myogenic factors, myogenin (MyoG), myocyte enhancer factor 2C (Mef2c) and myosin heavy chain (MyHC, p < 0.05). These changes resulted in accelerated muscle differentiation from myoblasts to myotubes (MyHC-positive area +56.52%; myotube width +34.08% vs. Veh, p < 0.05). In C2C12 myotubes, a palmitate-mediated decrease in the phosphorylation of forkhead box protein O1A (FOXO1A) and increase in the expression of E3 ubiquitin ligases, atrogin-1 and muscle RING-finger protein 1 (MuRF1) were reversed by HD6277 (p < 0.05). Additionally, HD6277 inhibited palmitate-induced apoptotic events such as the Bcl-2 (Bcl2)-associated X protein (Bax)/Bcl-2 ratio, caspase 3 cleavage and nuclear fragmentation in C2C12 myoblasts and myotubes (p < 0.05). These beneficial HD6277-mediated actions disappeared after the addition of an Akt inhibitor (p < 0.05). Similar to in vitro studies, HD6277 administration in obese and aged mice increased myogenic factors and decreased E3 ubiquitin ligase expression and apoptotic events (p < 0.05). HD6277 increased muscle strength (+9.88% vs. Aged, p < 0.05) and myofiber thickness (+29.01% vs. Aged, p < 0.05) in aging mice but only improved myofiber thickness (+11.84% vs. HFD, p < 0.05) in obese mice.
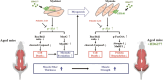
Conclusion: HD6277 can increase myogenic factors and reduce E3 ligase-mediated proteolysis to inhibit muscle atrophy in aged mice. Our results suggest that GPR40 agonists may have potential as therapeutic agents for sarcopenia.
Keywords: GPR40; muscle atrophy; myoblast; myogenic factors; myotube; sarcopenia.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have nothing to report.
The authors declare no conflicts of interest.
Figures






References
-
- Cruz‐Jentoft A. J. and Sayer A. A., “Sarcopenia,” Lancet 393 (2019): 2636–2646. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

