Circulating Food Allergen-Specific Antibodies, Beyond IgG4, Are Elevated in Eosinophilic Esophagitis
- PMID: 40230181
- PMCID: PMC12515539
- DOI: 10.1111/cea.70055
Circulating Food Allergen-Specific Antibodies, Beyond IgG4, Are Elevated in Eosinophilic Esophagitis
Abstract
Introduction: Eosinophilic esophagitis (EoE) is a chronic inflammatory condition with an incompletely understood immuno-pathogenesis involving a T2 response. EoE is triggered by food allergens although, unlike IgE-mediated allergies, it exhibits high IgG4 levels in oesophageal biopsies and in circulation. We investigated whether other antibody isotypes specific for food allergens are elevated in EoE and vary with disease activity.
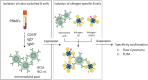
Methods: Plasma samples from patients with active EoE (n = 51), inactive EoE (n = 82) and non-EoE controls (n = 14) were analysed for food-specific IgG and IgA subclasses against casein, whey, wheat, egg and individual cow's milk allergens by ELISA. α-lactalbumin (Bos d 4)- and β-lactoglobulin (Bos d 5)-specific B cells were measured by flow cytometry in a subset of patients.
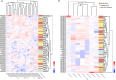
Results: Food allergen-specific antibodies in the plasma varied across EoE subgroups and non-EoE controls. Elevated IgG4 in EoE patients confirmed a strong antibody response to food allergens, including casein, wheat and egg. αS1-casein (Bos d 9)-specific IgG, IgG2, IgG4, IgA1 and IgA2 differed between EoE and non-EoE controls and between active and inactive EoE. β-casein (Bos d 11, A1 variant) measurements showed higher levels of specific IgG2 and IgG4 in both EoE groups, whereas whey-derived allergens showed opposing responses: Bos d 4 responses favoured IgG4, and Bos d 5 responses were elevated across multiple IgG and IgA subclasses in EoE. Allergen-specific B cells could not be isolated from the circulation.
Conclusion: Our findings reveal distinct antibody profiles in EoE plasma, with elevated IgG and IgA subclasses beyond IgG4, highlighting a complex immune response to food allergens. Differential antibody responses support their clinical relevance in dietary management strategies, while the absence of allergen-specific B cells in circulation likely restricts antibody production to the inflamed oesophagus. Future research should explore whether these antibody profiles can guide personalised treatment and novel therapeutic targets in EoE.
Keywords: allergen‐specific antibodies; eosinophilic esophagitis; humoural response.
© 2025 The Author(s). Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
M.A. has received research grants from the Swiss National Science Foundation (310,030_201,053/1), European Union (EU CURE, EU Syn‐Air‐G), is the Co‐Chair for EAACI Scientific Program, is on the Advisory Boards of Stanford University Sean Parker Asthma Allergy Center (CA, USA), LEO Foundation Skin Immunology Research Center Copenhagen, Denmark. P.S. received fees for consulting and grants from Falk Pharma, Takeda, Sanofi, AbbVie, Janssen‐Cilag, Ferring, Pfizer, Galapagos and Lilly. A.S. has consultant contracts with Astra Zeneca, BMS‐Receptos, Calypso, EsoCap, Falk Pharma, GSK, Pfizer, Sanofi‐Regeneron and Shire. A.M.S. has received speaker fees from Abbvie, BMS, Dr. Falk Pharma, GSK, Pfizer, Sanofi‐Regeneron, none of which are relevant for this work. L.B. declares fees for advisory from AbbVie, Amgen, BMS, Falk, Janssen, Pfizer, Lilly, Takeda, Sanofi, Esocap and speaker fees for Takeda, Sanofi, Abbvie, Lilly, Falk, BMS, Pfizer none of which are relevant for this work. W.V. has consultant contracts with Mabylon A.G. and received research grants from the Promedica Stiftung, the Swiss EoE Foundation and Novartis Research Foundation.
Figures




References
-
- Cook D., Zala A., Bollipo S., Potter M. D., Walker M. M., and Talley N. J., “Oesophageal Food Bolus Obstruction and Eosinophilic Oesophagitis,” Internal Medicine Journal 49, no. 8 (2019): 1032–1034. - PubMed
-
- Navarro P., Arias Á., Arias‐González L., Laserna‐Mendieta E. J., Ruiz‐Ponce M., and Lucendo A. J., “Systematic Review With Meta‐Analysis: The Growing Incidence and Prevalence of Eosinophilic Oesophagitis in Children and Adults in Population‐Based Studies,” Alimentary Pharmacology & Therapeutics 49, no. 9 (2019): 1116–1125. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

