Clinical and plasma proteomic characterization of heart failure with supranormal left ventricular ejection fraction: An emerging entity of heart failure
- PMID: 40230291
- PMCID: PMC12482836
- DOI: 10.1002/ejhf.3654
Clinical and plasma proteomic characterization of heart failure with supranormal left ventricular ejection fraction: An emerging entity of heart failure
Abstract
Aims: The clinical guidelines categorize heart failure (HF) based on left ventricular ejection fraction (LVEF). However, the current LVEF cutoffs, 40% and 50%, may not fully address the underlying characteristics and cardiovascular risk of HF, particularly for HF with higher LVEF. This study aimed to characterize HF with supranormal ejection fraction (HFsnEF) using different LVEF cutoffs (35%, 55%, and 70% for men, and 40%, 60%, and 75% for women).
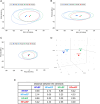
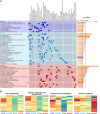
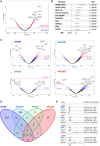
Methods and results: This study divided 442 patients from the CHART-Omics study into four groups: HF with reduced ejection fraction (HFrEF) (n = 55, 65.5 years), HF with mildly reduced ejection fraction (HFmrEF) (n = 125, 69.3 years), HF with normal ejection fraction (HFnEF) (n = 215, 69.0 years) and HFsnEF (n = 47, 67.1 years). When clinical backgrounds were adjusted and HFnEF served as the reference, HFsnEF carried an increased hazard ratio (HR) for the composite of cardiovascular death and HF hospitalization of 2.71 (95% confidence interval [CI] 1.10-6.66, p = 0.030), while HFrEF had a HR of 3.14 (95% CI 1.36-7.23, p = 0.007). HFsnEF was characterized by an increase in relative left ventricular wall thickness and a decrease in left ventricular dimensions, whereas increased left ventricular mass and dimensions characterized HFrEF. Quantitative analysis of 4670 plasma proteins showed essential differences between HFsnEF and HFrEF, for example, 'protein synthesis' versus 'cell morphology', 'cellular assembly and organization' and 'nucleic acid metabolism' for underlying pathophysiology, and 'energy production' versus 'connective tissue disorders' and 'cell-to-cell signalling and interaction' for prognostication.
Conclusions: Heart failure with supranormal ejection fraction, an unnoticed but emerging entity in HF, carries a similarly increased cardiovascular risk as HFrEF but has unique structural and plasma proteomic characteristics.
Keywords: Cardiac structure; Cardiovascular risk; Classification; Heart failure; Left ventricular ejection fraction; Proteomics.
© 2025 The Author(s). European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures


References
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. 10.1002/ejhf.2333 - DOI - PubMed
-
- Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352–380. 10.1002/ejhf.2115 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

