Targeting the CD39/CD73 pathway: New insights into cardiac fibrosis and inflammation in female cardiac surgery patients
- PMID: 40230374
- PMCID: PMC11994921
- DOI: 10.1016/j.jmccpl.2025.100294
Targeting the CD39/CD73 pathway: New insights into cardiac fibrosis and inflammation in female cardiac surgery patients
Abstract
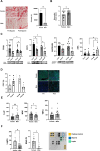
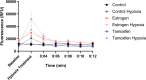
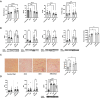
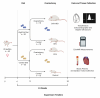
Women undergoing cardiac surgery suffer from worse outcomes than their male counterparts. The reasons for this disparity are multifactorial, but the loss of the protective effects of estrogen likely plays a role. Estrogen acts on the CD39/CD73 purine pathway, and loss of estrogen effects may contribute to the increased inflammation seen in post-menopausal women. We aimed to compare CD39/CD73 expression and downstream fibrosis, and inflammation in men and women undergoing cardiac surgery and then used an ovariectomy/high fat diet mouse model to approximate women who present for cardiac surgery to test therapeutics. We found decreased CD39 and CD73 in women compared to men, which was associated with increased fibrosis. Apyrase supplementation (a CD39 mimetic) improved ejection fraction and decreased E/e'. Increased CD73 function (via dipyridamole) decreased fibrosis. This study demonstrates the importance of purinergic dysfunction in cardiovascular disease in women and presents two potential therapeutics to improve cardiac health via manipulation of purine pathways.
Keywords: Apyrase; CD39; CD73; Cardiac surgery; Diastolic function; Fibrosis.
© 2025 Published by Elsevier Ltd.
Conflict of interest statement
Robina Matyal reports financial support was provided by National Institutes of Health. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- EUGenMed Cardiovascular Clinical Study Group, Regitz-Zagrosek V., Oertelt-Prigione S., Prescott E., Franconi F., Gerdts E., et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37:24–34. doi: 10.1093/eurheartj/ehv598. - DOI - PubMed
-
- Mosca L., Benjamin E.J., Berra K., Bezanson J.L., Dolor R.J., Lloyd-Jones D.M., et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–1423. doi: 10.1016/j.jacc.2011.02.005. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

