Signaling Pathways Involved in Acute Pancreatitis
- PMID: 40230438
- PMCID: PMC11995411
- DOI: 10.2147/JIR.S485804
Signaling Pathways Involved in Acute Pancreatitis
Abstract
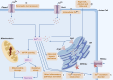
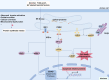
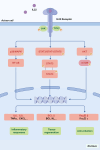
Acute pancreatitis (AP) is a common digestive emergency with high morbidity and mortality. Over the past decade, significant progress has been made in understanding the mechanisms of AP, including oxidative stress, disruptions in calcium homeostasis, endoplasmic reticulum stress, inflammatory responses, and various forms of cell death. This review provides an overview of the typical signaling pathways involved and proposes the latest clinical translation prospects. These strategies are important for the early management of AP, preventing multi-organ injury, and improving the overall prognosis of the disease.
Keywords: acute pancreatitis; calcium overload; cell death; endoplasmic reticulum stress; signaling pathway.
© 2025 Luo et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures
Similar articles
-
Mitochondrial Dysfunction, Through Impaired Autophagy, Leads to Endoplasmic Reticulum Stress, Deregulated Lipid Metabolism, and Pancreatitis in Animal Models.Gastroenterology. 2018 Feb;154(3):689-703. doi: 10.1053/j.gastro.2017.10.012. Epub 2017 Oct 23. Gastroenterology. 2018. PMID: 29074451 Free PMC article.
-
New insights into regulatory cell death and acute pancreatitis.Heliyon. 2023 Jul 7;9(7):e18036. doi: 10.1016/j.heliyon.2023.e18036. eCollection 2023 Jul. Heliyon. 2023. PMID: 37519748 Free PMC article. Review.
-
Drug D, a Diosgenin Derive, Inhibits L-Arginine-Induced Acute Pancreatitis through Meditating GSDMD in the Endoplasmic Reticulum via the TXNIP/HIF-1α Pathway.Nutrients. 2022 Jun 22;14(13):2591. doi: 10.3390/nu14132591. Nutrients. 2022. PMID: 35807771 Free PMC article.
-
Inhibition of endoplasmic reticulum stress by 4-phenylbutyric acid prevents vital organ injury in rat acute pancreatitis.Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G838-G847. doi: 10.1152/ajpgi.00102.2018. Epub 2018 Aug 23. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 30138574
-
New insights into acute pancreatitis.Nat Rev Gastroenterol Hepatol. 2019 Aug;16(8):479-496. doi: 10.1038/s41575-019-0158-2. Nat Rev Gastroenterol Hepatol. 2019. PMID: 31138897 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

