The role of CAFs in therapeutic resistance in triple negative breast cancer: an emerging challenge
- PMID: 40230452
- PMCID: PMC11994926
- DOI: 10.3389/fmolb.2025.1568865
The role of CAFs in therapeutic resistance in triple negative breast cancer: an emerging challenge
Abstract
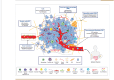
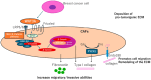
The tumor microenvironment (TME) is a crucial element of cancerous tissue and has emerged as a promising target for therapeutic strategies. The complex variety of stromal cells within the TME plays a vital role in determining the tumor's aggressiveness and its resistance to treatment. Tumor progression is not solely driven by cancer cells harboring genetic mutations but is also significantly influenced by non-cancerous host cells within the TME, which strongly impact tumor growth, metastasis, and the response to therapies. Cancer-associated fibroblasts (CAFs) are a diverse group of stromal cells within the TME. They play dual roles, both promoting and inhibiting tumor growth, making them intriguing targets for enhancing cancer therapies. Their significant contribution to creating a tumor-supportive environment has diminished the effectiveness of various cancer treatments, including radiation, chemotherapy, immunotherapy, and hormone therapy. Research has increasingly focused on understanding how CAFs contribute to therapy resistance in triple-negative breast cancer (TNBC) to improve treatment outcomes. However, the ways in which CAF patterns affect the TME and the response to immunotherapy in TNBC are not yet well understood and the interactions between CAFs, tumor cells, and immune cells in TNBC remain largely unexplored. In this review, we thoroughly exam ine the relationship between TNBC progression and CAF patterns. We discuss the current understanding of CAF heterogeneity, their role in tumor progression, and their impact on the tumor's response to therapeutic agents in TNBC. Additionally, we explore the potential and possible strategies for therapies targeting CAFs.
Keywords: CAF; TME; TNBC; cancer therapies; therapeutic resistance.
Copyright © 2025 Brogna, Varone, DelSesto and Ferrara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cancer-associated fibroblasts: The chief architect in the tumor microenvironment.Front Cell Dev Biol. 2023 Jan 30;11:1089068. doi: 10.3389/fcell.2023.1089068. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36793444 Free PMC article. Review.
-
Bruceine D suppresses CAF-promoted TNBC metastasis under TNF-α stimulation by inhibiting Notch1-Jagged1/NF-κB(p65) signaling.Phytomedicine. 2024 Jan;123:154928. doi: 10.1016/j.phymed.2023.154928. Epub 2023 Jun 16. Phytomedicine. 2024. PMID: 38043386
-
Tumor microenvironment and immunotherapy for triple-negative breast cancer.Biomark Res. 2024 Dec 31;12(1):166. doi: 10.1186/s40364-024-00714-6. Biomark Res. 2024. PMID: 39741315 Free PMC article. Review.
-
Characterization of cancer-associated fibroblasts (CAFs) and development of a CAF-based risk model for triple-negative breast cancer.Cancer Cell Int. 2023 Nov 25;23(1):294. doi: 10.1186/s12935-023-03152-w. Cancer Cell Int. 2023. PMID: 38007443 Free PMC article.
-
Therapeutic Targeting of Stromal-Tumor HGF-MET Signaling in an Organotypic Triple-Negative Breast Tumor Model.Mol Cancer Res. 2022 Jul 6;20(7):1166-1177. doi: 10.1158/1541-7786.MCR-21-0317. Mol Cancer Res. 2022. PMID: 35348758 Free PMC article.
Cited by
-
Targeting CAF-specific metabolic pathways in breast cancer.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul 1. doi: 10.1007/s00210-025-04390-7. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40590919 Review.
References
-
- Bejarano L., Jordāo M. J. C., Joyce J. A. (2021). Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11 (4), 933–959. PMID: 33811125. 10.1158/2159-8290.CD-20-1808 - DOI - PubMed
-
- Broad R. V., Jones S. J., Teske M. C., Wastall L. M., Hanby A. M., Thorne J. L., et al. (2021). Inhibition of interferon-signalling halts cancer-associated fibroblast-dependent protection of breast cancer cells from chemotherapy. Br. J. Cancer 124 (6), 1110–1120. 10.1038/s41416-020-01226-4 - DOI - PMC - PubMed
-
- Buyukhatipoglu H., Babacan T., Kertmen N., Balakan O., Suner A., Ates O., et al. (2015). A retrospective analysis of adjuvant CAF, AC-T and TAC regimens in triple negative early stage breast cancer. J. BUON 20 (1), 22–27. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

