Clinical Outcomes for Patients With Ulcerative Colitis in Cases of Withdrawal and Resumption of Janus Kinase Inhibitors: Multicenter Cohort Study
- PMID: 40230498
- PMCID: PMC11995396
- DOI: 10.1093/crocol/otaf020
Clinical Outcomes for Patients With Ulcerative Colitis in Cases of Withdrawal and Resumption of Janus Kinase Inhibitors: Multicenter Cohort Study
Abstract
Background: Janus kinase inhibitors (JAKis) have revolutionized ulcerative colitis (UC) management; however, the consequences of treatment discontinuation in patients achieving clinical remission remain poorly understood. This study investigated the clinical outcomes following JAKi discontinuation and retreatment effectiveness in patients with relapse.
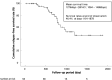
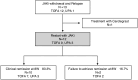
Methods: In this multicenter retrospective cohort study, we analyzed 101 patients with UC who received their first JAKi treatment between 2018 and 2024. Among them, 53 who achieved remission (Patient-Reported Outcome 2 = 0) in week 8 were included. The primary endpoint was a comparison of relapse-free survival between the treatment continuation and discontinuation groups (n = 37 and 16, respectively). The secondary endpoints included assessment of post-discontinuation remission maintenance and post-retreatment remission rates.
Results: The proportion of female patients in the discontinuation group (68.8%) was higher (P = .0478) than the continuation group (40.5%). The mean relapse-free survival was significantly longer in the continuation group than in the discontinuation group (1679 vs 882 days, cumulative relapse-free rate 83.3% vs 13.6%, P < .001, respectively). In the latter, 13 patients experienced relapse during follow-up (post-discontinuation mean relapse-free survival: 326 days), although all patients remained in clinical and biological remission. Notably, among patients who received JAKi retreatment, 83.3% achieved remission in week 8.
Conclusions: To our knowledge, this is the first real-world study to evaluate the effects of JAKi discontinuation on the outcomes for patients with UC. JAKi discontinuation in patients in remission was associated with a high relapse risk. JAKi retreatment was highly effective in patients who experienced relapse after treatment discontinuation, providing valuable evidence for managing treatment interruption.
Keywords: JAK inhibitors; relapse; remission; treatment discontinuation; ulcerative colitis.
© The Author(s) 2025. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
M.N. received grants from AbbVie GK, Alfresa Pharma Corporation, KYORIN Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharmaceutical Co., Ltd., and MIYARISAN Pharmaceutical Co. Ltd. and lecture fees from EA Pharma Co., Ltd., Gilead Science Inc., Takeda Pharmaceutical Co., Ltd., AbbVie GK, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharmaceutical Co., Ltd., JIMRO Co., Ltd., KYORIN Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., and Pfizer Co., Ltd, outside of the submitted work.
Figures




Similar articles
-
Oral Janus kinase inhibitors for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2020 Jan 27;1(1):CD012381. doi: 10.1002/14651858.CD012381.pub2. Cochrane Database Syst Rev. 2020. PMID: 31984480 Free PMC article.
-
Effectiveness of janus kinase inhibitors in relapsing giant cell arteritis in real-world clinical practice and review of the literature.Arthritis Res Ther. 2024 Jun 5;26(1):116. doi: 10.1186/s13075-024-03314-9. Arthritis Res Ther. 2024. PMID: 38840219 Free PMC article. Review.
-
Relapse Rates and Predictors for Relapse in Ulcerative Colitis and Crohn's Disease Patients After Discontinuation of Vedolizumab or Ustekinumab: The REVEUS Study.J Clin Med. 2025 Mar 7;14(6):1793. doi: 10.3390/jcm14061793. J Clin Med. 2025. PMID: 40142602 Free PMC article.
-
Evolution After Anti-TNF Discontinuation in Patients With Inflammatory Bowel Disease: A Multicenter Long-Term Follow-Up Study.Am J Gastroenterol. 2017 Jan;112(1):120-131. doi: 10.1038/ajg.2016.569. Epub 2016 Dec 13. Am J Gastroenterol. 2017. PMID: 27958281
-
Impact of Prior Biologic or Janus Kinase Inhibitor Therapy on Efficacy and Safety of Etrasimod in the ELEVATE UC 52 and ELEVATE UC 12 Trials.J Crohns Colitis. 2024 Nov 4;18(11):1780-1794. doi: 10.1093/ecco-jcc/jjae079. J Crohns Colitis. 2024. PMID: 38877972 Free PMC article. Clinical Trial.
References
-
- Sandborn WJ, Su C, Sands BE, et al.; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723-1736. doi: https://doi.org/10.1056/NEJMoa1606910 - DOI - PubMed
-
- Feagan BG, Danese S, LoftusEV, Jr, et al.Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397(10292):2372-2384. doi: https://doi.org/10.1016/S0140-6736(21)00666-8 - DOI - PubMed
-
- Danese S, Vermeire S, Zhou W, et al.Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi: https://doi.org/10.1016/S0140-6736(22)00581-5 - DOI - PubMed
-
- Ytterberg SR, Bhatt DL, Mikuls TR, et al.; ORAL Surveillance Investigators. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316-326. doi: https://doi.org/10.1056/NEJMoa2109927 - DOI - PubMed
-
- Panés J, D’Haens GR, Sands BE, et al.Analysis of tofacitinib safety in ulcerative colitis from the completed global clinical developmental program up to 9.2 years of drug exposure. United European Gastroenterol J. 2024;12(6):793-801. doi: https://doi.org/10.1002/ueg2.12584 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
