Risk-based in silico mutagenic assessment of benzodiazepine impurities using three QSAR tools
- PMID: 40230516
- PMCID: PMC11995136
- DOI: 10.1016/j.toxrep.2025.102008
Risk-based in silico mutagenic assessment of benzodiazepine impurities using three QSAR tools
Abstract
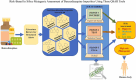
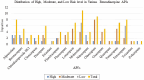
Benzodiazepines, widely prescribed psychoactive drugs, may contain DNA-reactive (mutagenic) impurities formed during synthesis, posing significant health risks. Owing to animal testing requirements, traditional in vitro and in vivo methods for assessing mutagenicity are time-consuming, costly, and ethically challenging. Computational approaches, particularly in silico (Q)SAR models, provide an efficient alternative for predicting toxicity based on chemical structure. This study evaluated the mutagenic potential of 88 benzodiazepine-related impurities using three freely accessible (Q)SAR tools: TOXTREE (Ames Test Alert by ISS), Toxicity Estimation Software Tool (TEST) with nearest neighbour and consensus models, and VEGA, a QSAR tool that integrates multiple mutagenicity prediction models, including the CAESAR Ames Mutagenicity Model. The tools were validated using a dataset of 99 chemicals with known Ames test results. TOXTREE exhibited the highest sensitivity (80.7 %) and accuracy (72.2 %) for predicting mutagenicity, whereas VEGA and TEST provided balanced accuracy (66.2 % and 66.7 %, respectively) and high specificity (74.5 % and 76.6 %, respectively). The risk assessment categorised 21 impurities as high risk, 11 as moderate-high risk, 28 as moderate-low risk, 22 as low risk, and 6 as equivocal, with expert review finalising classifications. The findings emphasise the integration of multiple (Q)SAR tools for early mutagenicity detection, regulatory compliance, and reduced reliance on animal testing. Further refinement of predictive models and additional computational approaches are recommended to enhance the accuracy of the risk assessment.
Keywords: (Q)SAR tools; Benzodiazepines; In silico evaluation; Mutagenic impurities; Regulatory implications.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Predicting the mutagenic potential of chemicals in tobacco products using in silico toxicology tools.Toxicol Mech Methods. 2020 Nov;30(9):672-678. doi: 10.1080/15376516.2020.1805836. Epub 2020 Aug 26. Toxicol Mech Methods. 2020. PMID: 32752976
-
An assessment of mutagenicity of chemical substances by (quantitative) structure-activity relationship.Genes Environ. 2020 Jul 2;42:23. doi: 10.1186/s41021-020-00163-1. eCollection 2020. Genes Environ. 2020. PMID: 32626544 Free PMC article. Review.
-
Improvement of quantitative structure-activity relationship (QSAR) tools for predicting Ames mutagenicity: outcomes of the Ames/QSAR International Challenge Project.Mutagenesis. 2019 Mar 6;34(1):3-16. doi: 10.1093/mutage/gey031. Mutagenesis. 2019. PMID: 30357358 Free PMC article.
-
Validation of Toxtree and SciQSAR in silico predictive software using a publicly available benchmark mutagenicity database and their applicability for the qualification of impurities in pharmaceuticals.Regul Toxicol Pharmacol. 2013 Nov;67(2):285-93. doi: 10.1016/j.yrtph.2013.08.008. Epub 2013 Aug 19. Regul Toxicol Pharmacol. 2013. PMID: 23969001
-
Risk Assessment, Detection and Control of Mutagenic Impurities in Pharmaceuticals: Emphasis on Nitrosamines.Crit Rev Anal Chem. 2025 Jun 17:1-42. doi: 10.1080/10408347.2025.2517357. Online ahead of print. Crit Rev Anal Chem. 2025. PMID: 40525553 Review.
Cited by
-
MXene based nanoarchitectures for organic contaminants degradation under sonophotocatalytic environment: eco-friendly synthesis, catalytic attributes and recent advancements.RSC Adv. 2025 Aug 7;15(34):28093-28120. doi: 10.1039/d5ra04096e. eCollection 2025 Aug 1. RSC Adv. 2025. PMID: 40778104 Free PMC article. Review.
References
-
- Allen M.J., Sabir S., Sharma S. GABA Recept. 2024 〈https://www.ncbi.nlm.nih.gov/books/NBK526124/〉 PMID: 30252380.
-
- Jewett B.E., Sharma S. Physiol., GABA. 2024 PMID: 30020683 〈https://www.ncbi.nlm.nih.gov/books/NBK513311/〉.
-
- Campbell J.M., Grinias K., Facchine K., Igne B., Clawson J., Peterson J., Wolters A., Barry J., Watson S., Leach K. Analysis of unstable degradation impurities of a benzodiazepine and their quantification without isolation using multiple linear regression. J. Pharm. Biomed. Anal. 2019 Apr 15;167:1–6. doi: 10.1016/j.jpba.2019.01.028. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

