Combination therapy with alisertib enhances the anti-tumor immunity induced by a liver cancer vaccine
- PMID: 40230537
- PMCID: PMC11995041
- DOI: 10.1016/j.isci.2025.112120
Combination therapy with alisertib enhances the anti-tumor immunity induced by a liver cancer vaccine
Abstract
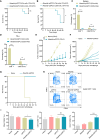
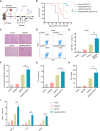
Alisertib is a potent aurora A kinase inhibitor in clinical trials for cancer treatment, but its efficacy on cancer vaccines remains unclear. Here, we developed a DNA vaccine targeting glypican-3 (pGPC3) and evaluated its efficacy with alisertib in hepatocellular carcinoma (HCC) models. The combination therapy of pGPC3 vaccine and alisertib significantly inhibited subcutaneous tumor growth, enhanced the induction and maturation of CD11c+ and CD8+CD11c+ dendritic cells (DCs), and expanded tumor-specific CD8+ T cell responses. CD8+ T cell depletion abolished the anti-tumor effects, underscoring the essential role of functional CD8+ T cell responses. Moreover, the combined treatment promoted memory CD8+ T cell induction, providing long-term protection. In liver orthotopic tumor models, the combination of pGPC3 vaccine and alisertib demonstrated potent therapeutic efficacy through CD8+ T cell responses. These results indicate that alisertib enhances the pGPC3 vaccine's therapeutic effect, offering a promising strategy for HCC treatment.
Keywords: Biological sciences; Cancer systems biology; Immunology; Natural sciences; Systems biology.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
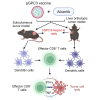
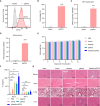
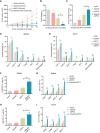
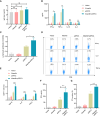
Similar articles
-
Co-immunization with L-Myc enhances CD8+ or CD103+ DCs mediated tumor-specific multi-functional CD8+ T cell responses.Cancer Sci. 2021 Sep;112(9):3469-3483. doi: 10.1111/cas.15044. Epub 2021 Jul 10. Cancer Sci. 2021. PMID: 34157192 Free PMC article.
-
Co-immunization with IFI35 enhances the therapeutic effect of an adenovirus vaccine against renal carcinoma.Int J Biol Macromol. 2025 Jan;286:138515. doi: 10.1016/j.ijbiomac.2024.138515. Epub 2024 Dec 6. Int J Biol Macromol. 2025. PMID: 39647736
-
Therapeutic Adenovirus Vaccine Combined Immunization with IL-12 Induces Potent CD8+ T Cell Anti-Tumor Immunity in Hepatocellular Carcinoma.Cancers (Basel). 2022 Sep 17;14(18):4512. doi: 10.3390/cancers14184512. Cancers (Basel). 2022. PMID: 36139670 Free PMC article.
-
Scientific Rationale Supporting the Clinical Development Strategy for the Investigational Aurora A Kinase Inhibitor Alisertib in Cancer.Front Oncol. 2015 Aug 24;5:189. doi: 10.3389/fonc.2015.00189. eCollection 2015. Front Oncol. 2015. PMID: 26380220 Free PMC article. Review.
-
An update on the pharmacokinetics and pharmacodynamics of alisertib, a selective Aurora kinase A inhibitor.Clin Exp Pharmacol Physiol. 2016 Jun;43(6):585-601. doi: 10.1111/1440-1681.12571. Clin Exp Pharmacol Physiol. 2016. PMID: 26999067 Review.
References
-
- Baharom F., Ramirez-Valdez R.A., Khalilnezhad A., Khalilnezhad S., Dillon M., Hermans D., Fussell S., Tobin K.K.S., Dutertre C.A., Lynn G.M., et al. Systemic vaccination induces CD8(+) T cells and remodels the tumor microenvironment. Cell. 2022;185:4317–4332.e15. doi: 10.1016/j.cell.2022.10.006. - DOI - PMC - PubMed
-
- Lopes A., Bastiancich C., Bausart M., Ligot S., Lambricht L., Vanvarenberg K., Ucakar B., Gallez B., Préat V., Vandermeulen G. New generation of DNA-based immunotherapy induces a potent immune response and increases the survival in different tumor models. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-001243. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

