Anti-CTLA-and anti-PD-1 immune checkpoint inhibitor antibodies impair human sperm motility in-vitro
- PMID: 40230698
- PMCID: PMC11994717
- DOI: 10.3389/fphar.2025.1534975
Anti-CTLA-and anti-PD-1 immune checkpoint inhibitor antibodies impair human sperm motility in-vitro
Abstract
Background: Immune checkpoint inhibitors (ICIs), namely, anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) monoclonal antibody Ipilimumab and anti- and programmed cell death 1 (PD-1) monoclonal antibodies Nivolumab, and Pembrolizumab, have improved the treatment outcomes for many other cancer types. However, their impact on fertility remains under-explored.
Methods: The possible direct effects of ICIs on human sperm was investigated. Spermatozoa from ten normozoospermic donors were exposed to Ipilimumab, Nivolumab, or Pembrolizumab at concentrations ranging from 1 to 100 ng/mL. Sperm motility was assessed through standard laboratory process. Cell viability and apoptosis markers were evaluated by flow-cytometry using fluorescent Annexin-V probe and Terminal Uridine Nick-End Label (TUNEL) assays. Protein-A-purified therapeutic antibodies (IgG) were also evaluated.
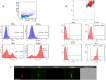
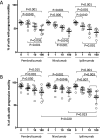
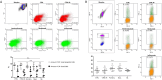
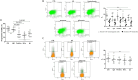
Results: Spermatozoa had high PD-1 (>99%) and negligible CTLA-4 expression. Exposure to ICIs, was associated with a concentration-dependent impairment of sperm motility, noticeable for Pembrolizumab and Ipilimumab since 10 ng/mL, and for Nivolumab since 100 ng/mL. However, no significant effect on cell apoptosis or viability was shown. Purified IgG from ICIs maintained the adverse effect on cell motility without affecting viability.
Conclusion: ICIs, specifically Pembrolizumab, Nivolumab, and Ipilimumab, adversely affect human sperm motility in vitro. Further research is required to understand the underlying mechanisms and clinical implications.
Keywords: Annexin-V; Nivolumab; Pembrolizumab; TUNEL; ipilimumab.
Copyright © 2025 Cosci, De Toni, Del Fiore, Di Nisio, Carraro, Radu, Bertazza, Mocellin, Pigozzo, Crivellaro, Coppola and Ferlin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
Similar articles
-
Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors.J Immunother Cancer. 2021 Feb;9(2):e001945. doi: 10.1136/jitc-2020-001945. J Immunother Cancer. 2021. PMID: 33563773 Free PMC article.
-
Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology.Ann Pharmacother. 2015 Aug;49(8):907-37. doi: 10.1177/1060028015586218. Epub 2015 May 19. Ann Pharmacother. 2015. PMID: 25991832 Review.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Ophthalmic Immune-Related Adverse Events after Anti-CTLA-4 or PD-1 Therapy Recorded in the American Academy of Ophthalmology Intelligent Research in Sight Registry.Ophthalmology. 2021 Jun;128(6):910-919. doi: 10.1016/j.ophtha.2020.11.001. Epub 2020 Nov 6. Ophthalmology. 2021. PMID: 33166553
-
Ileus in patients treated with immune checkpoint inhibitors: A retrospective, pharmacovigilance study using Food and Drug Administration Adverse Event Reporting System database.Pharmacoepidemiol Drug Saf. 2022 Nov;31(11):1199-1205. doi: 10.1002/pds.5493. Epub 2022 Jul 6. Pharmacoepidemiol Drug Saf. 2022. PMID: 35689298
References
-
- Barroso-Sousa R., Barry W. T., Garrido-Castro A. C., Hodi F. S., Min L., Krop I. E., et al. (2018). Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 4, 173–182. 10.1001/jamaoncol.2017.3064 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

