Efficacy of Tislelizumab in Lung Cancer Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 40230743
- PMCID: PMC11995441
- DOI: 10.7759/cureus.80609
Efficacy of Tislelizumab in Lung Cancer Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
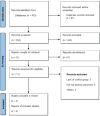
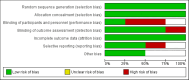
This systematic review and meta-analysis evaluated the efficacy of tislelizumab, alone or in combination with chemotherapy, in patients with lung cancer. A comprehensive literature search was conducted across PubMed, Embase, Web of Science, and CENTRAL databases until February 15, 2025. Only randomized controlled trials (RCTs) comparing tislelizumab with control treatments in lung cancer patients were included. The primary outcomes assessed were overall survival (OS) and progression-free survival (PFS). Four phase-III RCTs involving 1,837 patients were included in the analysis. The results demonstrated that tislelizumab significantly improved OS (hazard ratios (HR): 0.72, 95% CI: 0.63-0.81) and PFS (HR: 0.61, 95% CI: 0.54-0.68) compared to control treatments. Subgroup analyses revealed consistent benefits across both non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) populations, with no significant differences between cancer types. Similarly, the efficacy of tislelizumab was comparable whether administered as monotherapy or in combination with chemotherapy. Low heterogeneity was observed among the included studies, suggesting consistency in treatment effects. Follow-up duration across studies ranged from 14.2 to 16.7 months. These findings indicate that tislelizumab, either alone or combined with chemotherapy, is an effective treatment option for lung cancer patients, demonstrating significant improvements in survival outcomes. However, further high-quality RCTs are needed to validate these results, particularly in SCLC patients, where evidence is limited to a single study. Future research should also consider patient-specific factors such as age, gender, and comorbidities to refine treatment strategies.
Keywords: immunotherapy; lung cancer; meta-analysis; pd-1 inhibitors; tislelizumab.
Copyright © 2025, Ul Bassar et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures






Similar articles
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Immune checkpoint inhibitors versus chemotherapy as second-line therapy for advanced oesophageal squamous cell carcinoma: a systematic review and economic evaluation.Therap Adv Gastroenterol. 2024 Feb 28;17:17562848241233134. doi: 10.1177/17562848241233134. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 38425370 Free PMC article. Review.
-
The safety and efficacy of tislelizumab, alone or in combination with chemotherapy, for the treatment of non-small cell lung cancer: a systematic review of clinical trials.BMC Pulm Med. 2023 Dec 8;23(1):495. doi: 10.1186/s12890-023-02755-3. BMC Pulm Med. 2023. PMID: 38066549 Free PMC article.
-
Comparative efficacy of immune checkpoint inhibitors combined with chemotherapy in patients with advanced driver-gene negative non-small cell lung cancer: A systematic review and network meta-analysis.Heliyon. 2024 May 7;10(10):e30809. doi: 10.1016/j.heliyon.2024.e30809. eCollection 2024 May 30. Heliyon. 2024. PMID: 38774326 Free PMC article.
Cited by
-
Vitamin D and Immune Checkpoint Inhibitors in Lung Cancer: A Synergistic Approach to Enhancing Treatment Efficacy.Int J Mol Sci. 2025 May 9;26(10):4511. doi: 10.3390/ijms26104511. Int J Mol Sci. 2025. PMID: 40429656 Free PMC article. Review.
References
-
- 69 - Cancer of the lung: Non-small cell lung cancer and small cell lung cancer. Araujo LH, Horn L, Merritt RE, Shilo K, Xu-Welliver M, Carbone DP. Abeloff's Clin Oncol. 2020:1108–1158.
-
- A review on lung cancer. Jyothi T, Sowjanya M, Yerikala R, Chandra YP, Venugopalaiah P. J Multidisciplinary Res. 2025;5:1–6.
-
- Recent advances in lung cancer research: unravelling the future of treatment. Bertolaccini L, Casiraghi M, Uslenghi C, Maiorca S, Spaggiari L. Updates Surg. 2024;76:2129–2140. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
