The Shock of Shatter: Understanding Silique and Silicle Dehiscence for Improving Oilseed Crops in Brassicaceae
- PMID: 40230828
- PMCID: PMC11994477
- DOI: 10.1002/pld3.70058
The Shock of Shatter: Understanding Silique and Silicle Dehiscence for Improving Oilseed Crops in Brassicaceae
Abstract
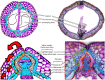
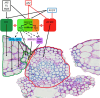
Silique dehiscence, despite being an essential physiological process for seed dispersal for dehiscent fruits, is disadvantageous for the agricultural industry. While crops have been selected against the expression of natural, spontaneous shattering to protect the seeds for harvest, fruit dehiscence in the field can be promoted through abiotic factors such as wind, drought, and hail that can be detrimental in reducing crop yield and profitability. In crops like canola, pennycress, and Camelina, this impact could be as high as 50%, creating economic losses for both the industry and the economy. Mitigating the effects of fruit dehiscence is crucial to prevent seed loss, economic loss, and the persistence of volunteer plants, which interfere with crop rotation and require increased weed control. Developing agronomic traits through genetic manipulation to enhance the strength of the fruiting body can prevent seed dispersal mechanisms from occurring and boost yield efficiency and preservation. Current research into this area has created mutant plants with indehiscent fruits by reducing allele function that determines the identity of the various anatomical layers of the fruit. Future genetic approaches may focus on strengthening siliques by enhancing secondary cell walls through either increased lignification or reducing cell wall-degrading enzymes to achieve shatter tolerance. This review focuses on improving our knowledge within members of the Brassicaceae family to create a better understanding of silique/silicle dehiscence for researchers to establish a groundwork for broader applications across diverse crops. This knowledge will directly lead to improved agricultural productivity and ensure a stable food supply, addressing global challenges the world is facing.
Keywords: agriculture production; cell wall; lignification; oilseed crops; silique dehiscence; yield increases.
© 2025 The Author(s). Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Alberta Canola . 2021. “Canola Statistics.” https://albertacanola.com/about/canola‐statistics/.
-
- Alonso‐Cantabrana, H. , Ripoll J. J., Ochando I., Vera A., Ferrándiz C., and Martínez‐Laborda A.. 2007. “Common Regulatory Networks in Leaf and Fruit Patterning Revealed by Mutations in the Arabidopsis ASYMMETRIC LEAVES1 Gene.” Development 134: 2663–2671. - PubMed
-
- Alvarez, J. , and Smyth D. R.. 1997. “Carpel Development Genes in ‘Arabidopsis’.” Flowering Newsletter 23: 12–17.
-
- Alvarez, J. , and Smyth D. R.. 2002. “ CRABS CLAW and SPATULA Genes Regulate Growth and Pattern Formation During Gynoecium Development in Arabidopsis thaliana .” International Journal of Plant Sciences 163: 17–41.
Publication types
LinkOut - more resources
Full Text Sources

