IFN-γ-Induced intestinal epithelial cell-type-specific programmed cell death: PANoptosis and its modulation in Crohn's disease
- PMID: 40230837
- PMCID: PMC11994596
- DOI: 10.3389/fimmu.2025.1523984
IFN-γ-Induced intestinal epithelial cell-type-specific programmed cell death: PANoptosis and its modulation in Crohn's disease
Abstract
Background: Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) and is considered a Th1-mediated disease, supported by the over-expression of interferon-gamma (IFN-γ) in the intestinal lamina propria. IFN-γ has a pleiotropic effect on the intestinal epithelial cells (IECs), suggesting that IFN-γ-induced responses may differ between epithelial cell types.
Methods: We established human small intestinal organoids (enteroids) derived from non-IBD controls and CD patients. Using human enteroids, the major response of IECs induced by IFN-γ was evaluated, focusing on the IFN-γ-induced programmed cell death (PCD) pathway. Identified IFN-γ-induced responses were validated in surgically resected intestinal samples and publicly available single-cell RNA-sequencing datasets.
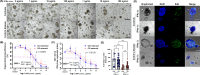
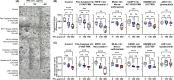
Results: IFN-γ stimulated programmed cell death (PCD) of IECs in both control and CD enteroids in a dose-dependent manner. Pyroptosis, apoptosis. and necroptosis were activated in enteroids, suggesting that PANoptosis was the main process of IFN-γ-induced PCD in IECs. The response to IFN-γ depends on the cell type of the IECs. IFN-γ induced depletion of enterocytes with upregulation of PANoptosis-associated genes, while leading to expansion of goblet cells without significant change in PANoptosis-associated gene expression. Individual PCD inhibitors were insufficient to block IFN-γ-induced cytotoxicity, whereas the selective JAK1 inhibitor (upadacitinib) effectively blocked IFN-γ-induced cytotoxicity and PANoptosis. Furthermore, PANoptosis was significantly activated in surgically resected tissues and in publicly available single-cell RNA-sequencing datasets of intestinal tissues from patients with CD.
Conclusion: IFN-γ induces PANoptosis in enterocytes, which can be treated with a selective JAK1 inhibitor in patients with CD.
Keywords: Crohn’s disease; PANoptosis; enteroid; interferon-gamma; intestinal epithelial cell; intestinal organoid; programmed cell death; selective JAK1 inhibitor.
Copyright © 2025 Lee, Kim, Cha, Moon, Kim, Chang, Kim and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
PRDM5 promotes the apoptosis of epithelial cells induced by IFN-γ during Crohn's disease.Pathol Res Pract. 2017 Jun;213(6):666-673. doi: 10.1016/j.prp.2016.12.004. Epub 2016 Dec 5. Pathol Res Pract. 2017. PMID: 28476379
-
TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells.Cell Death Dis. 2021 Sep 23;12(10):864. doi: 10.1038/s41419-021-04151-3. Cell Death Dis. 2021. PMID: 34556638 Free PMC article.
-
Interleukin-28A induces epithelial barrier dysfunction in CD patient-derived intestinal organoids.Am J Physiol Gastrointest Liver Physiol. 2021 May 1;320(5):G689-G699. doi: 10.1152/ajpgi.00064.2020. Epub 2021 Feb 17. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33595362
-
PANoptosis in intestinal epithelium: its significance in inflammatory bowel disease and a potential novel therapeutic target for natural products.Front Immunol. 2025 Jan 7;15:1507065. doi: 10.3389/fimmu.2024.1507065. eCollection 2024. Front Immunol. 2025. PMID: 39840043 Free PMC article. Review.
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

