Bivalent circular RNA vaccines against porcine epidemic diarrhea virus and transmissible gastroenteritis virus
- PMID: 40230842
- PMCID: PMC11994721
- DOI: 10.3389/fimmu.2025.1562865
Bivalent circular RNA vaccines against porcine epidemic diarrhea virus and transmissible gastroenteritis virus
Abstract
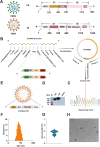
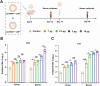
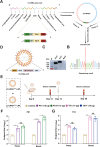
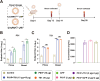
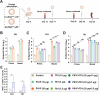
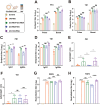
Porcine Epidemic Diarrhea Virus (PEDV) and Transmissible Gastroenteritis Virus (TGEV) pose significant threats to neonatal piglets, leading to severe diarrhea and potentially lethal consequences. Beyond enforcing stringent biosecurity protocols, effective and safe vaccinations are crucial in mitigating the impact of these diseases. In this study, the PEDV S1 (PS1) and TGEV S1 (TS1) antigens were initially chosen as candidates for the development of circRNA vaccines. Recognizing the comparatively lower immunogenicity of the PS1 antigen in contrast to the TS1 antigen, we strategically conjugated the PS1 with the pig fragment crystallizable (Fc) region to form PS1F. Despite these efforts, the bivalent circRNA vaccine prepared using an equal amount of the circRNAPS1F and circRNATS1 mixture still led to a reduction in the antibody levels against PS1. Subsequent dosage optimization of these two circRNA vaccines resulted in the induction of comparable levels of antigen specific antibodies and T cell immunity. Furthermore, sequential vaccination regimen with bivalent circRNA vaccine and commercial inactivated vaccines (IAV) could elicit a predominantly Th1-driven antibody responses and effectively neutralize both PEDV and TGEV. Our findings not only provide a potential strategy for the development of bivalent or multivalent circRNA/mRNA-based vaccines but also highlight the promising application of sequential vaccination strategies within the swine industry.
Keywords: bivalent; circRNA vaccine; porcine epidemic diarrhea virus; sequential vaccination; transmissible gastroenteritis virus.
Copyright © 2025 Zhang, Wang, Chu, Ma, Gao, Wu, Qiao, Wang, Zhao, Hu, Li, Zhang, Song, Yu, Wang, Dong and Liu.
Conflict of interest statement
Author HH was employed by the company Ankerui Shanxi Biological Cell Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Construction of a bivalent DNA vaccine co-expressing S genes of transmissible gastroenteritis virus and porcine epidemic diarrhea virus delivered by attenuated Salmonella typhimurium.Virus Genes. 2016 Jun;52(3):354-64. doi: 10.1007/s11262-016-1316-z. Epub 2016 Mar 15. Virus Genes. 2016. PMID: 26980672
-
Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus.PLoS One. 2013;8(3):e57468. doi: 10.1371/journal.pone.0057468. Epub 2013 Mar 19. PLoS One. 2013. PMID: 23526943 Free PMC article.
-
A trivalent enteric coronaviruses inactivated vaccine provides effective protection against PEDV, TGEV, and PDCoV.Vet Microbiol. 2025 Sep;308:110630. doi: 10.1016/j.vetmic.2025.110630. Epub 2025 Jul 4. Vet Microbiol. 2025. PMID: 40633274
-
Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts.Virus Res. 2016 Dec 2;226:93-107. doi: 10.1016/j.virusres.2016.05.016. Epub 2016 May 19. Virus Res. 2016. PMID: 27212686 Free PMC article. Review.
-
Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses.Vet Microbiol. 2017 Jul;206:45-51. doi: 10.1016/j.vetmic.2016.11.029. Epub 2016 Dec 2. Vet Microbiol. 2017. PMID: 27964998 Free PMC article. Review.
References
-
- Pasick J, Berhane Y, Ojkic D, Maxie G, Embury-Hyatt C, Swekla K, et al. . Investigation into the role of potentially contaminated feed as a source of the first-detected outbreaks of porcine epidemic diarrhea in Canada. Transbound Emerg Dis. (2014) 61(5):397–410. doi: 10.1111/tbed.12269 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

