The protective effect of Chlorella vulgaris against diclofenac toxicity in Clarias gariepinus: haemato-immunological parameters and spleen histological features as outcome markers
- PMID: 40230852
- PMCID: PMC11994428
- DOI: 10.3389/fimmu.2025.1566496
The protective effect of Chlorella vulgaris against diclofenac toxicity in Clarias gariepinus: haemato-immunological parameters and spleen histological features as outcome markers
Abstract
Introduction: Diclofenac (DCF) is a commonly utilized medication in the non-steroidal anti-inflammatory drug category that is released into aquatic systems in significant amounts. Chlorella vulgaris (C. vulgaris) is rich in active phytochemicals known for their haemato-immunological boosting properties.
Methods: Our objective was to investigate the haemato-immunological protective properties of Chlorella in mitigating the toxic effects of DCF. Five groups of Clarias gariepinus, each comprising 36 fish, were assigned over a two-week period. The groups were assigned as follows: control group, which received a basal diet only; DCF1 group, which received a basal diet and was exposed to 20 μg/L of DCF; DCF2 group, which received a basal diet and was exposed to 10 mg/L of DCF; and Chlorella +DCF1 and Chlorella+DCF2 groups, which were exposed to the same DCF doses as Groups 2 and 3, respectively, while also being fed a diet containing 25% Chlorella.
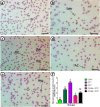
Results: Exposure to both doses of DCF significantly decreased erythrocyte count, hemoglobin content, white blood cell count, phagocytic index, and lysozyme activity, while increased eosinophil and neutrophil % in an equipotent manner. The low dose caused a more pronounced reduction in packed cell volume (PCV)% and large lymphocyte% compared to the high dose. A significant decline in platelet count was observed only with the low DCF dose, while the high dose led to a decrease in monocyte%. DCF intoxication led to a dose-related decrease in small lymphocyte% and an increase in erythrocyte morphological alterations and interleukin (IL)-6 levels. The DCF2 group exhibited a higher increase in apoptotic RBCs than the DCF1 group. Intervention with Chlorella alongside the two DCF doses significantly normalized RBC count and eosinophil %, increased PCV% and small lymphocyte%, and decreased erythrocyte abnormalities to an equal extent. Large lymphocyte% in the Chlorella+DCF1 group was successfully restored to normal levels. Phagocytic index and lysozyme activity in the supplemented groups were lower, while IL-6 levels were higher than in the DCF groups. The percentage of apoptotic cells decreased with Chlorella administration, with the Chlorella+DCF1 group showing fewer apoptotic cells than the Chlorella+DCF2 group. Histopathological deterioration and excessive collagen deposition were observed in the spleen of DCF groups, while notable improvements were seen following C. vulgaris supplementation.
Conclusion: These findings suggest that dietary inclusion of C. vulgaris may antagonize the haemato-cytological abnormalities induced by DCF intoxication.
Keywords: african catfish; haemato-immunology; microalgae; non-steroidal anti-inflammatory drug; physiology; spleen.
Copyright © 2025 Gabr, Mohamed, Abou Khalil and Sayed.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The protective effects of dietary microalgae against hematological, biochemical, and histopathological alterations in pyrogallol-intoxicated Clarias gariepinus.Heliyon. 2024 Dec 5;10(24):e40930. doi: 10.1016/j.heliyon.2024.e40930. eCollection 2024 Dec 30. Heliyon. 2024. PMID: 39759355 Free PMC article.
-
Chronic diclofenac (DCF) exposure alters both enzymatic and haematological profile of African catfish, Clarias gariepinus.Drug Chem Toxicol. 2015 Oct;38(4):383-90. doi: 10.3109/01480545.2014.974108. Epub 2014 Nov 4. Drug Chem Toxicol. 2015. PMID: 25367777
-
Dietary Moringa oleifera mitigates Fluconazole-Induced immunological and spleen-histological alterations in Catfish (Clarias gariepinus).BMC Vet Res. 2024 Jul 18;20(1):325. doi: 10.1186/s12917-024-04173-x. BMC Vet Res. 2024. PMID: 39026256 Free PMC article.
-
Fishmeal replacement with Spirulina Platensis and Chlorella vulgaris in African catfish (Clarias gariepinus) diet: Effect on antioxidant enzyme activities and haematological parameters.Res Vet Sci. 2018 Aug;119:67-75. doi: 10.1016/j.rvsc.2018.05.013. Epub 2018 May 25. Res Vet Sci. 2018. PMID: 29864632
-
Botanical remedies ameliorate oxidative stress, immune response, and histopathology in Clarias gariepinus exposed to pyrogallol.Environ Pollut. 2025 Oct 1;382:126628. doi: 10.1016/j.envpol.2025.126628. Epub 2025 Jun 4. Environ Pollut. 2025. PMID: 40480506
References
-
- Williams RT, Cook JC. Exposure to pharmaceuticals present in the environment. Ther Innov Regul Sci. (2007) 41:133–41. doi: 10.1177/009286150704100202 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

