Development of a refined experimental mouse model of myasthenia gravis with anti-acetylcholine receptor antibodies
- PMID: 40230859
- PMCID: PMC11994731
- DOI: 10.3389/fimmu.2025.1521382
Development of a refined experimental mouse model of myasthenia gravis with anti-acetylcholine receptor antibodies
Abstract
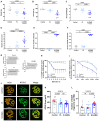
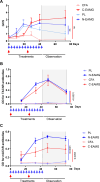
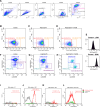
Myasthenia gravis (MG) is an autoimmune disorder primarily caused by autoantibodies that target the acetylcholine receptor (AChR) at the neuromuscular junction (NMJ). The classical experimental autoimmune myasthenia gravis (C-EAMG) mouse model has long been used by immunizing mice with acetylcholine receptor from Torpedo fish (T-AChR), combined with complete Freund's adjuvant (CFA). This mixture is administered via subcutaneous injections into the hind footpads and back, but CFA often causes strong inflammatory reactions, including lesions at the injection sites. Our objective was to develop a new EAMG model (N-EAMG) that is more compliant with animal welfare. C57Bl/6 mice were immunized twice weekly by intraperitoneal (i.p.) injection of T-AChR with a poly(I:C) and lipopolysaccharide (LPS) adjuvant mix. Control mice were injected with either physiological saline or the adjuvant mix alone. Various doses and injection schedules were tested, and the new model was compared with C-EAMG. Clinical symptoms were scored, antibody subtypes against T-AChR and mouse AChR were measured, and NMJ morphology and functionality were evaluated. We demonstrate that the N-EAMG model is at least as effective as the C-EAMG model. Moreover, similar to the C-EAMG model, the N-EAMG model is characterized by the production of T-AChR and m-AChR antibodies. This model also exhibited alterations in transmission at the NMJ due to antibody attack, resulting in a decrease in AChR surface area and increased AChR fragmentation. Symptoms were similar in both models but appeared more rapidly in the N-EAMG model. In addition, investigating the sensitization mechanism, we showed that i.p. injections of T-AChR with the poly(I:C)/LPS adjuvant mix, led to the recruitment in monocytes and changes in the two peritoneal macrophage subpopulations that were able to phagocytose T-AChR. These observations suggest that macrophage subtypes, albeit with varying efficiency, present the T-AChR to immune cells, leading to a specific immune response and the development of anti-AChR antibodies. In conclusion, our results demonstrate that this novel EAMG model is as effective as the C-EAMG model and offers several advantages. In particular, this model is more suitable for animal welfare and can replace the classical model in preclinical and fundamental research.
Keywords: LPS; adjuvant; autoimmunity; experimental model; myasthenia gravis; poly(I:C); refinement.
Copyright © 2025 You, Lippens, Fayet, Maillard, Betemps, Grondin, Vilquin, Dragin and Le Panse.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Immunization with Recombinantly Expressed LRP4 Induces Experimental Autoimmune Myasthenia Gravis in C57BL/6 Mice.Immunol Invest. 2017 Jul;46(5):490-499. doi: 10.1080/08820139.2017.1299754. Epub 2017 Apr 4. Immunol Invest. 2017. PMID: 28375749
-
Novel animal models of acetylcholine receptor antibody-related myasthenia gravis.Ann N Y Acad Sci. 2012 Dec;1274:133-9. doi: 10.1111/j.1749-6632.2012.06773.x. Ann N Y Acad Sci. 2012. PMID: 23252908 Review.
-
Injection of inactive Bordetella pertussis and complete Freund's adjuvant with Torpedo californica AChR increases the occurrence of experimental autoimmune myasthenia gravis in C57BL/6 mice.Autoimmunity. 2017 Aug;50(5):293-305. doi: 10.1080/08916934.2017.1329831. Epub 2017 May 26. Autoimmunity. 2017. PMID: 28548588
-
Acetylcholine receptor-induced experimental myasthenia gravis: what have we learned from animal models after three decades?Arch Immunol Ther Exp (Warsz). 2012 Feb;60(1):19-30. doi: 10.1007/s00005-011-0158-6. Epub 2011 Dec 8. Arch Immunol Ther Exp (Warsz). 2012. PMID: 22159475 Review.
-
Animal models of myasthenia gravis.Clin Immunol. 2000 Feb;94(2):75-87. doi: 10.1006/clim.1999.4807. Clin Immunol. 2000. PMID: 10637092 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

