Isolation and Characterization of a Novel Lytic Phage N22 and Its Effect on Drug-Resistant Klebsiella Pneumoniae
- PMID: 40231317
- PMCID: PMC11995918
- DOI: 10.2147/IDR.S515363
Isolation and Characterization of a Novel Lytic Phage N22 and Its Effect on Drug-Resistant Klebsiella Pneumoniae
Abstract
Background: Klebsiella pneumoniae (KP) infections present a significant clinical challenge and are frequently associated with elevated drug resistance. The use of phage therapy has resurged in response to escalating antibiotic resistance. This study aimed to address the multidrug resistance crisis in intensive care units by exploring the use of ceftazidime/avibactam (CAZ/AVI), a widely used clinical antimicrobial agent, in conjunction with phage therapy.
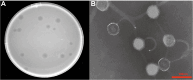
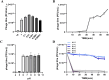
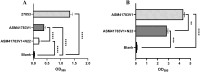
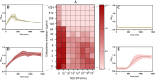
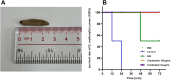
Materials and methods: We screened a clinical strain of KP from ICU and successfully isolated phage N22 from hospital wastewater. We conducted an in-depth analysis of the physiological and biochemical properties of phage N22 and determined its optimal multiplicity of infection with the clinical KP strain. The inhibitory effects of phage N22 in combination with CAZ/AVI on biofilm formation were investigated. Comparative efficacies of these combinations were evaluated using a Galleria mellonella (G. mellonella) model.
Results: Phage N22 inhibited KP biofilm formation. The impact of varying phage N22 concentrations when used alongside CAZ/AVI was examined, and the combination of phage N22 and CAZ/AVI was more effective against KP than CAZ/AVI alone.
Conclusion: This study provides a preliminary investigation into the effects of combining CAZ/AVI with phage therapy, highlighting its potential significance in developing novel therapeutic strategies for bacterial infections resistant to CAZ/AVI. The findings underscore the importance of advancing highly effective phage agents as alternative treatment modalities for patients with infections refractory to conventional antibiotics.
Keywords: Klebsiella pneumoniae; ceftazidime/avibactam; drug-resistant; phage therapy.
© 2025 Liu et al.
Conflict of interest statement
No potential conflict of interest to be declared by the author(s).
Figures
Similar articles
-
Ceftazidime-avibactam resistance in KPC-producing Klebsiella pneumoniae accompanied hypermucoviscosity acquisition.BMC Microbiol. 2024 Oct 28;24(1):439. doi: 10.1186/s12866-024-03508-w. BMC Microbiol. 2024. PMID: 39468460 Free PMC article.
-
Ceftazidime-avibactam in the treatment of infections from carbapenem-resistant Klebsiella pneumoniae: Ceftazidime-avibactam against CR-KP infections.J Glob Antimicrob Resist. 2021 Sep;26:20-25. doi: 10.1016/j.jgar.2021.04.022. Epub 2021 May 18. J Glob Antimicrob Resist. 2021. PMID: 34020072
-
Treating complicated carbapenem-resistant enterobacteriaceae infections with ceftazidime/avibactam: a retrospective study with molecular strain characterisation.Int J Antimicrob Agents. 2017 Jun;49(6):770-773. doi: 10.1016/j.ijantimicag.2017.01.018. Epub 2017 Apr 4. Int J Antimicrob Agents. 2017. PMID: 28389354
-
Efficacy and Safety of Ceftazidime-Avibactam for the Treatment of Carbapenem-Resistant Enterobacterales Bloodstream Infection: a Systematic Review and Meta-Analysis.Microbiol Spectr. 2022 Apr 27;10(2):e0260321. doi: 10.1128/spectrum.02603-21. Epub 2022 Apr 4. Microbiol Spectr. 2022. PMID: 35377233 Free PMC article.
-
Update of clinical application in ceftazidime-avibactam for multidrug-resistant Gram-negative bacteria infections.Infection. 2022 Dec;50(6):1409-1423. doi: 10.1007/s15010-022-01876-x. Epub 2022 Jul 4. Infection. 2022. PMID: 35781869 Review.
References
LinkOut - more resources
Full Text Sources

