Orthogonal photoswitching of heterobivalent azobenzene glycoclusters: the effect of glycoligand orientation in bacterial adhesion
- PMID: 40231321
- PMCID: PMC11995721
- DOI: 10.3762/bjoc.21.57
Orthogonal photoswitching of heterobivalent azobenzene glycoclusters: the effect of glycoligand orientation in bacterial adhesion
Abstract
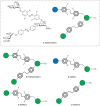
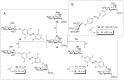
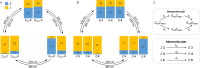
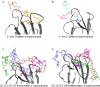
Carbohydrate recognition is fundamental to a plethora of cellular processes and hence the elucidation of the structural determinants of the recognition process is a prerequisite for understanding and manipulating carbohydrate-protein interactions, such as in the inhibition of carbohydrate-specific bacterial adhesion. For receptor binding, glycoligands have to be properly oriented in three-dimensional space and additionally, secondary interactions exerted by multivalent glycoligands have an effect on affinity. A recently introduced orthogonally photoswitchable heterobivalent azobenzene Glc/Man glycocluster was utilized to examine these aspects of carbohydrate recognition in a bacterial adhesion-inhibition assay. The measured results were systematically contextualized employing new reference compounds such as the respective homobivalent Man/Man glycocluster. An in-depth study comprising the analysis of the photochromic properties and the potential as inhibitors of bacterial adhesion of the synthetic glycophotoswitches in their different isomeric states led to new insights into the role of ligand orientation in carbohydrate recognition. The experimental results were underpinned by molecular modeling.
Keywords: FimH; azobenzene glycoconjugates; carbohydrate recognition; docking; orthogonal photoswitching.
Copyright © 2025, Friedrich and Lindhorst.
Figures


References
LinkOut - more resources
Full Text Sources
