Hexadehydro Diels-Alder/Alkynyliodanation Cascade: A Highly Regioselective Entry to Polycyclic Aromatics
- PMID: 40231630
- PMCID: PMC12038833
- DOI: 10.1021/acs.orglett.5c00956
Hexadehydro Diels-Alder/Alkynyliodanation Cascade: A Highly Regioselective Entry to Polycyclic Aromatics
Abstract
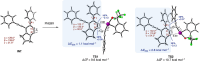
We report here a cascade process integrating the hexadehydro Diels-Alder (HDDA) reaction with alkynyliodanation, enabling efficient synthesis of highly substituted aryl-λ3-iodanes. Heating a mixture of a tetrayne and an alkynylbenziodoxole induces regioselective insertion of the tetrayne-derived aryne into the alkynyl-iodine(III) bond, yielding a 1,4-dialkynyl-2-iodanyl-3-aryl(or alkyl)benzene derivative. The unique regiochemistry facilitates subsequent π-extension, allowing divergent access to polyaromatic frameworks, such as helicenes and cyclopenta[cd]pyrenes, underscoring the utility of aryne carboiodanation in complex aromatic synthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Iodonium(III) Ylide: An Iodoalkylation Reagent with Aryne.J Org Chem. 2025 Feb 14;90(6):2372-2385. doi: 10.1021/acs.joc.4c02892. Epub 2025 Feb 5. J Org Chem. 2025. PMID: 39910405
-
Access to Fully Substituted Dihydroindazoles Via Hexadehydro-Diels-Alder/[3 + 2] Cycloaddition.J Org Chem. 2023 Oct 20;88(20):14736-14747. doi: 10.1021/acs.joc.3c01901. Epub 2023 Oct 11. J Org Chem. 2023. PMID: 37819716
-
Highly Conjugated π-Systems Arising from Cannibalistic Hexadehydro-Diels-Alder Couplings: Cleavage of C-C Single and Triple Bonds.Chemistry. 2020 Dec 4;26(68):15989-16000. doi: 10.1002/chem.202002511. Epub 2020 Oct 29. Chemistry. 2020. PMID: 32619049 Free PMC article.
-
Hexadehydro-Diels-Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes.Chem Rev. 2021 Feb 24;121(4):2413-2444. doi: 10.1021/acs.chemrev.0c00825. Epub 2021 Jan 25. Chem Rev. 2021. PMID: 33492939 Free PMC article. Review.
-
Mechanistic and Kinetic Factors of ortho-Benzyne Formation in Hexadehydro-Diels-Alder (HDDA) Reactions.Chemistry. 2021 May 26;27(30):7978-7991. doi: 10.1002/chem.202100608. Epub 2021 May 2. Chemistry. 2021. PMID: 33783896 Free PMC article. Review.
Cited by
-
Iodine(III)-Mediated Cyclization Cascade of Diynes to Benzofulvene Derivatives.Org Lett. 2025 Aug 29;27(34):9559-9564. doi: 10.1021/acs.orglett.5c03133. Epub 2025 Aug 17. Org Lett. 2025. PMID: 40820503 Free PMC article.
References
-
- Hexadehydro Diels–Alder/Alkynyliodanation Cascade: A Highly Regioselective and Expedient Entry to Polycyclic Aromatics. ChemRxiv 2025, 10.26434/chemrxiv-2025-w5hqm. - DOI
-
- Fluegel L. L.; Hoye T. R. Hexadehydro-Diels-Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes. Chem. Rev. 2021, 121, 2413–2444. 10.1021/acs.chemrev.0c00825. - DOI - PMC - PubMed
- Holden C.; Greaney M. F. The Hexadehydro-Diels-Alder Reaction: A New Chapter in Aryne Chemistry. Angew. Chem., Int. Ed. 2014, 53, 5746–5749. 10.1002/anie.201402405. - DOI - PMC - PubMed
- Kim N.; Choi M.; Suh S.-E.; Chenoweth D. M. Aryne Chemistry: Generation Methods and Reactions Incorporating Multiple Arynes. Chem. Rev. 2024, 124, 11435–11522. 10.1021/acs.chemrev.4c00296. - DOI - PubMed
-
- Mitake A.; Nagai R.; Sekine A.; Takano H.; Sugimura N.; Kanyiva K. S.; Shibata T. Consecutive HDDA and TDDA Reactions of Silicon-Tethered Tetraynes for the Synthesis of Dibenzosilole-Fused Polycyclic Compounds and Their Unique Reactivity. Chem. Sci. 2019, 10, 6715–6720. 10.1039/C9SC00960D. - DOI - PMC - PubMed
- Xiao X.; Hoye T. R. One-Pot, Three-Aryne Cascade Strategy for Naphthalene Formation from 1,3-Diynes and 1,2-Benzdiyne Equivalents. J. Am. Chem. Soc. 2019, 141, 9813–9818. 10.1021/jacs.9b04606. - DOI - PMC - PubMed
- Arora S.; Hoye T. R. Kobayashi Benzynes″ as Hexadehydro-Diels-Alder Diynophiles. Org. Lett. 2021, 23, 3349–3353. 10.1021/acs.orglett.1c00787. - DOI - PMC - PubMed
- Chinta B. S.; Lee D.; Hoye T. R. Coumarin (5,6-Benzo-2-Pyrone) Trapping of an HDDA-Benzyne. Org. Lett. 2021, 23, 2189–2193. 10.1021/acs.orglett.1c00342. - DOI - PMC - PubMed
- Lee D.; Ross S. P.; Xiao X.; Hoye T. R. Radial Hexadehydro-Diels-Alder Reactions. Chem. 2021, 7, 2527–2537. 10.1016/j.chempr.2021.08.010. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

