Nanoneedle-Based Electroporation for Efficient Manufacturing of Human Primary Chimeric Antigen Receptor Regulatory T-Cells
- PMID: 40231643
- PMCID: PMC12140303
- DOI: 10.1002/advs.202416066
Nanoneedle-Based Electroporation for Efficient Manufacturing of Human Primary Chimeric Antigen Receptor Regulatory T-Cells
Abstract
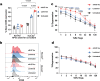
Regulatory T cells (Tregs) play a crucial role in moderating immune responses offering promising therapeutic options for autoimmune diseases and allograft rejection. Genetically engineering Tregs with chimeric antigen receptors (CARs) enhances their targeting specificity and efficacy. With non-viral transfection methods suffering from low efficiency and reduced cell viability, viral transduction is currently the only viable approach for GMP-compliant CAR-Treg production. However, viral transduction raises concerns over immunogenicity, insertional mutagenesis risk, and high costs, which limit clinical scalability. This study introduces a scalable nanoneedle electroporation (nN-EP) platform for GMP-compatible transfection of HLA-A2-specific CAR plasmids into primary human Tregs. The nN-EP system achieves 43% transfection efficiency, outperforming viral transduction at multiplicity of infection 1 by twofold. Importantly, nN-EP preserves Treg viability, phenotype and proliferative capacity. HLA-A2-specific CAR-Tregs generated using nN-EP show specific activation and superior suppressive function compared to polyclonal or virally transduced Tregs in the presence of HLA-A2 expressing antigen presenting cells. These findings underscore the potential of nN-EP as a GMP-suitable method for CAR-Treg production, enabling broader clinical application in immune therapies.
Keywords: CAR‐T; electroporation; nanoneedle; nanoscale electroporation; non‐viral transfection; regulatory T cells; tregs.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Giganti G., Atif M., Mohseni Y., Mastronicola D., Grageda N., Povoleri G. A., Miyara M., Scottà C., Eur. J. Immunol. 2021, 51, 39. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
