Immune-related adverse events-pembrolizumab-induced colitis-the importance of early diagnosis and treatment: A case report and review of the literature
- PMID: 40231646
- PMCID: PMC12033556
- DOI: 10.1177/03946320251326699
Immune-related adverse events-pembrolizumab-induced colitis-the importance of early diagnosis and treatment: A case report and review of the literature
Abstract
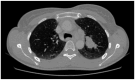
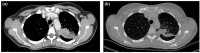
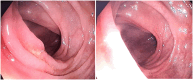
Immune Checkpoint Inhibitors (ICIs) are monoclonal antibodies that block inhibitory immune targets, such as cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death ligand 1 (PD-L). Pembrolizumab targets the PD-1 receptor of lymphocytes in lung cancer treatment. ICI checkpoint blockade enhances immunity against cancer cells. However, loss of immunoregulatory control can cause autoimmune reactions in various organs, leading to immune-related adverse events (irAEs). The most common irAE is ICIs-induced colitis, which usually develops 6-8 weeks after ICI initiation and can involve any part of the gastrointestinal system. Herein, we report a presentation of pembrolizumab-induced colitis in a female patient with metastatic lung cancer and review the most recent findings in the model of checkpoint-induced colitis. It was interesting to learn that the colon mucosa may show normal macroscopic findings, but microscopically, immunotherapy-induced autoimmune colitis could be present. Additionally, patients with grade 2 or higher symptoms should have a colonoscopy, receive systemic corticosteroids as treatment, and, based on their response, receive biologic therapy. Here, we present a case report of in a 45-year-old female who has been a smoker for 25 years, without comorbidities, and with metastatic lung cancer who developed colitis after the seventh cycle of pembrolizumab. This case presentation highlights the importance of early recognition and appropriate intervention in order to prevent permanent interruption of treatment with checkpoint inhibitors, as well as prevention of colitis complications.
Keywords: checkpoint inhibitor induced colitis; immune checkpoint inhibitors; immune-related adverse events; immunotherapy; non-small cell lung carcinoma; pembrolizumab.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Case report: reinitiating pembrolizumab treatment after small bowel perforation.BMC Cancer. 2019 Apr 24;19(1):379. doi: 10.1186/s12885-019-5577-5. BMC Cancer. 2019. PMID: 31018834 Free PMC article.
-
Improved survival and disease control following pembrolizumab-induced immune-related adverse events in high PD-L1 expressing non-small cell lung cancer with brain metastases.J Neurooncol. 2021 Mar;152(1):125-134. doi: 10.1007/s11060-020-03686-3. Epub 2021 Jan 7. J Neurooncol. 2021. PMID: 33415659 Free PMC article.
-
Compromise or not? A case report of successful treatment of pembrolizumab-induced hepatitis in a patient with non-small cell lung cancer with low-dose methylprednisolone and bicyclol.Thorac Cancer. 2020 Jul;11(7):2023-2030. doi: 10.1111/1759-7714.13463. Epub 2020 May 7. Thorac Cancer. 2020. PMID: 32379397 Free PMC article.
-
Tuberculosis following programmed cell death receptor-1 (PD-1) inhibitor in a patient with non-small cell lung cancer. Case report and literature review.Cancer Immunol Immunother. 2021 Apr;70(4):935-944. doi: 10.1007/s00262-020-02726-1. Epub 2020 Oct 17. Cancer Immunol Immunother. 2021. PMID: 33070259 Free PMC article. Review.
-
Durable Metastatic Melanoma Remission Following Pembrolizumab and Radiotherapy: A Case Report of Prophylactic Immunosuppression in a Patient with Myasthenia Gravis and Immune-Mediated Colitis.Front Immunol. 2021 Dec 8;12:788499. doi: 10.3389/fimmu.2021.788499. eCollection 2021. Front Immunol. 2021. PMID: 34956219 Free PMC article. Review.
References
-
- Lugowska I, Misale S, Califano R, et al. (2022) ESMO Handbook of Targeted Therapies and Precision. Brussels: ESMO Press, vol. 10, pp.124–125.
-
- Martins F, Sofiya L, Sykiotis GP, et al. (2019) Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nature Reviews Clinical Oncology 16: 563–580. - PubMed
-
- Schneider BJ, Naidoo J, Santomasso BD, et al. (2021) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. Journal of Clinical Oncology 39: 4073–4126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

