Coevolutionary interplay: Helminths-trained immunity and its impact on the rise of inflammatory diseases
- PMID: 40231720
- PMCID: PMC12002795
- DOI: 10.7554/eLife.105393
Coevolutionary interplay: Helminths-trained immunity and its impact on the rise of inflammatory diseases
Abstract
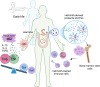
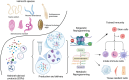
The gut biome, a complex ecosystem of micro- and macro-organisms, plays a crucial role in human health. A disruption in this evolutive balance, particularly during early life, can lead to immune dysregulation and inflammatory disorders. 'Biome repletion' has emerged as a potential therapeutic approach, introducing live microbes or helminth-derived products to restore immune balance. While helminth therapy has shown some promise, significant challenges remain in optimizing clinical trials. Factors such as patient genetics, disease status, helminth species, and the optimal timing and dosage of their products or metabolites must be carefully considered to train the immune system effectively. We aim to discuss how helminths and their products induce trained immunity as prospective to treat inflammatory and autoimmune diseases. The molecular repertoire of helminth excretory/secretory products (ESPs), which includes proteins, peptides, lipids, and RNA-carrying extracellular vesicles (EVs), underscores their potential to modulate innate immune cells and hematopoietic stem cell precursors. Mimicking natural delivery mechanisms like synthetic exosomes could revolutionize EV-based therapies and optimizing production and delivery of ESP will be crucial for their translation into clinical applications. By deciphering and harnessing helminth-derived products' diverse modes of action, we can unleash their full therapeutic potential and pave the way for innovative treatments.
Keywords: ESPs; EVs; excretory/secretory products; extracelular vesicles; gut biome; helminths; immunology; inflammation; inflammatory disorders; trained immunity.
© 2025, Carrera Silva et al.
Conflict of interest statement
EC, JP, AE No competing interests declared
Figures

Similar articles
-
Exosome-transported microRNAs of helminth origin: new tools for allergic and autoimmune diseases therapy?Parasite Immunol. 2015 Apr;37(4):208-14. doi: 10.1111/pim.12182. Parasite Immunol. 2015. PMID: 25712154 Review.
-
Therapeutic potential of helminths in autoimmune diseases: helminth-derived immune-regulators and immune balance.Parasitol Res. 2017 Aug;116(8):2065-2074. doi: 10.1007/s00436-017-5544-5. Epub 2017 Jun 29. Parasitol Res. 2017. PMID: 28664463 Review.
-
[Of worms and men--Administration of helminth products as an innovative approach to treatment of autoimmune diseases].Harefuah. 2015 Jul;154(7):428-31, 470, 469. Harefuah. 2015. PMID: 26380461 Review. Hebrew.
-
What Can Parasites Tell Us About the Pathogenesis and Treatment of Asthma and Allergic Diseases.Front Immunol. 2020 Sep 11;11:2106. doi: 10.3389/fimmu.2020.02106. eCollection 2020. Front Immunol. 2020. PMID: 33013887 Free PMC article. Review.
-
The hygiene theory harnessing helminths and their ova to treat autoimmunity.Clin Rev Allergy Immunol. 2013 Oct;45(2):211-6. doi: 10.1007/s12016-012-8352-9. Clin Rev Allergy Immunol. 2013. PMID: 23325330 Review.
References
-
- Alghanmi M, Minshawi F, Altorki TA, Zawawi A, Alsaady I, Naser AY, Alwafi H, Alsulami SM, Azhari AA, Hashem AM, Alhabbab R. Helminth-derived proteins as immune system regulators: a systematic review of their promise in alleviating colitis. BMC Immunology. 2024;25:21. doi: 10.1186/s12865-024-00614-2. - DOI - PMC - PubMed
-
- Arrais M, Maricoto T, Nwaru BI, Cooper PJ, Gama JMR, Brito M, Taborda-Barata L. Helminth infections and allergic diseases: Systematic review and meta-analysis of the global literature. The Journal of Allergy and Clinical Immunology. 2022;149:2139–2152. doi: 10.1016/j.jaci.2021.12.777. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- PICT-2021-I-A-00807/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (ANPCyT); Fondo para la Investigación Científica y Tecnológica (FONCYT), Argentina.
- Fiorini 2024/Fundación Florencio Fiorini, Argentina
- PIP 2022-0763/Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
LinkOut - more resources
Full Text Sources
Medical

