Heartbeat-related activity in the anterior thalamus differs between phasic and tonic REM sleep
- PMID: 40231737
- PMCID: PMC12072251
- DOI: 10.1113/JP287802
Heartbeat-related activity in the anterior thalamus differs between phasic and tonic REM sleep
Abstract
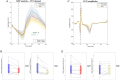
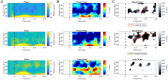
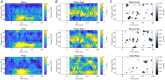
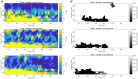
Rapid eye movement (REM) sleep is a fundamental sleep state associated with diverse functions from elemental physiological processes to higher order neurocognitive functions. A growing body of research indicates that REM sleep with eye movements (phasic REM) differs from REM periods without ocular activity (tonic) in terms of spontaneous and evoked neural responses. Studies using auditory stimulation consistently observed enhanced evoked responses in tonic versus phasic REM, indicating that external processing is largely diminished when the eyes move during REM sleep. Whereas exteroceptive processing during sleep is widely studied, investigations on interoception (the processing of bodily signals) during sleep are scarce, and limited to scalp electroencephalographic recordings. Here we studied interoceptive processing in a group of epileptic patients (N = 11) by measuring their heartbeat-related neural activity in the anterior nuclei of the thalamus (ANT) during phasic and tonic REM sleep and resting wakefulness. Evoked potentials and beta-low gamma spectral power locked to the heartbeat were significantly different in phasic REM compared with tonic REM and wakefulness. Heartbeat-related neural signals exhibited pronounced inter-trial phase synchronization at lower (7-20 Hz) oscillatory activity in all vigilance states, but reduced gamma synchronization at later time points in phasic REM only. Tonic REM and wakefulness did not show significant differences in heartbeat-related activity in the ANT. Our findings indicate that heartbeat-related neural activity is detectable at the level of the ANT, showing distinct signatures of interoceptive processing in phasic REM compared with tonic REM and wakefulness. KEY POINTS: We studied interoceptive processing in the anterior the thalamus (ANT). The ANT tracks cardiac signals during wakefulness and rapid eye movement (REM) sleep. Phasic REM shows distinct patterns of heartbeat-related oscillatory activity. Interoceptive processing might be attenuated during REM periods with eye movements.
Keywords: REM sleep; anterior thalamic nucleus; heartbeat; interoception; thalamography.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
No competing interests declared.
Figures
Similar articles
-
REM Sleep Microstates in the Human Anterior Thalamus.J Neurosci. 2021 Jun 30;41(26):5677-5686. doi: 10.1523/JNEUROSCI.1899-20.2021. Epub 2021 Apr 16. J Neurosci. 2021. PMID: 33863786 Free PMC article.
-
Cortical monitoring of cardiac activity during rapid eye movement sleep: the heartbeat evoked potential in phasic and tonic rapid-eye-movement microstates.Sleep. 2021 Sep 13;44(9):zsab100. doi: 10.1093/sleep/zsab100. Sleep. 2021. PMID: 33870427 Free PMC article.
-
Long-range alpha and beta and short-range gamma EEG synchronization distinguishes phasic and tonic REM periods.Sleep. 2018 Mar 1;41(3). doi: 10.1093/sleep/zsx210. Sleep. 2018. PMID: 29309685
-
The microstructure of REM sleep: Why phasic and tonic?Sleep Med Rev. 2020 Aug;52:101305. doi: 10.1016/j.smrv.2020.101305. Epub 2020 Mar 19. Sleep Med Rev. 2020. PMID: 32259697 Review.
-
Human alpha oscillations in wakefulness, drowsiness period, and REM sleep: different electroencephalographic phenomena within the alpha band.Neurophysiol Clin. 2002 Jan;32(1):54-71. doi: 10.1016/s0987-7053(01)00289-1. Neurophysiol Clin. 2002. PMID: 11915486 Review.
References
-
- Amici, R. , & Zoccoli, G. (2021). Physiological changes in the autonomic nervous system during sleep. In: Chokroverty S. & Cortelli P. (Eds.), Autonomic Nervous System and Sleep (pp. 43–50). Springer International Publishing, Cham. 10.1007/978-3-030-62263-3_5 - DOI
-
- Andrillon, T. , & Kouider, S. (2020). The vigilant sleeper: Neural mechanisms of sensory (de)coupling during sleep. Current Opinion in Physiology, Physiology of Sleep, 15, 47–59.
-
- Baird, B. , Tononi, G. , & LaBerge, S. (2022). Lucid dreaming occurs in activated rapid eye movement sleep, not a mixture of sleep and wakefulness. Sleep, 45(4), zsab294. - PubMed
-
- Bastuji, H. , & García‐Larrea, L. (1999). Evoked potentials as a tool for the investigation of human sleep. Sleep Medicine Reviews, 3(1), 23–45. - PubMed
MeSH terms
Grants and funding
- K_128117/National Research, Development and Innovation Office
- TKP2021-EGA-25/Ministry of Innovation and Technology
- TKP2021-NKTA-47/Ministry of Innovation and Technology
- János Bolyai/Magyar Tudományos Akadémia (MTA)
- ÚNKP-23-1/Innovációs és Technológiai Minisztérium (Ministry for Innovation and Technology)
LinkOut - more resources
Full Text Sources
