Pooled Analysis of 2 Randomized Clinical Trials to Evaluate the Efficacy and Safety of Clotrimazole 1% Otic Solution for the Treatment of Otomycosis in Adults
- PMID: 40231796
- PMCID: PMC12033508
- DOI: 10.1177/19160216251330629
Pooled Analysis of 2 Randomized Clinical Trials to Evaluate the Efficacy and Safety of Clotrimazole 1% Otic Solution for the Treatment of Otomycosis in Adults
Abstract
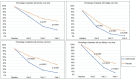
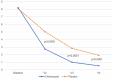
ImportanceThere is no antifungal otic drug for the treatment of otomycosis approved in the United States. Some current clotrimazole formulations in the market are used off-label.ObjectiveTo evaluate the efficacy and safety of clotrimazole 1% otic solution compared to placebo in treating otomycosis in adults.DesignTwo independent twin multicenter, randomized (2:1), double-blind, controlled clinical trials with identical designs were conducted from February 2020 to October 2021.SettingFifty-three sites located in the United States, Mexico, and Europe.ParticipantsAdults with uncomplicated otomycosis presented with symptoms, debris, and drainage clinically consistent with fungal infection.InterventionPatients received clotrimazole or placebo twice daily for 14 days and were evaluated on visit 1 (day 1), visit 2 (day 8-10), visit 3 (day 15), and follow-up visit 4 (day 24-26).Main Outcome MeasuresAt each visit, pruritus, otalgia, otorrhea, and ear fullness were assessed. Ear exudate was taken for a mycological and microbiological evaluation at baseline and, if present, at subsequent visits. The primary endpoint was a therapeutic cure (mycological and clinical) at visit 4 in the randomized population with positive fungal culture at baseline [mycological intent-to-treat (MITT)].ResultsThree hundred ninety-three patients received study medication (261 clotrimazole and 132 placebo). Efficacy data from the 228 patients (157 clotrimazole and 71 placebo) included in the MITT were analyzed. The clotrimazole group achieved a higher proportion for the primary endpoint than those with the placebo group (68.2% vs 25.4%; P < .0001), with a 42.8 difference in response rate (95% confidence interval: 30.3, 55.3). The treatment was safe and well tolerated, with 2.7% of related adverse events in the clotrimazole versus 1.5% in the placebo group.ConclusionsClotrimazole 1% otic solution has demonstrated its superiority over the placebo in each study and the pooled analysis. These are the results of the first international, multicenter clinical trials in which clotrimazole 1% otic solution demonstrates efficacy for the treatment of otomycosis.
Keywords: antifungal; clotrimazole; otic solution; otomycosis.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ansley J.F., Butehorn H.F., Todorov S., Tzvetkov V., Douglis F., Georgiev K., Moreira da Silva F.: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.Bernal-Sprekelsen: The author declared speaker honorarium for GSK Spain, Sanofi Spain, Consultant for Bionorica Germany.
Figures
Similar articles
-
Topical azole treatments for otomycosis.Cochrane Database Syst Rev. 2021 May 25;5(5):CD009289. doi: 10.1002/14651858.CD009289.pub2. Cochrane Database Syst Rev. 2021. PMID: 34033120 Free PMC article.
-
Efficacy and safety of eberconazole 1% otic solution compared to clotrimazole 1% solution in patients with otomycosis.Am J Otolaryngol. 2018 May-Jun;39(3):307-312. doi: 10.1016/j.amjoto.2018.03.017. Epub 2018 Mar 6. Am J Otolaryngol. 2018. PMID: 29551350 Clinical Trial.
-
Efficacy of topical clotrimazole vs. topical tolnaftate in the treatment of otomycosis. A randomized controlled clinical trial.Braz J Otorhinolaryngol. 2020 May-Jun;86(3):300-307. doi: 10.1016/j.bjorl.2018.12.007. Epub 2019 Feb 18. Braz J Otorhinolaryngol. 2020. PMID: 30826311 Free PMC article. Clinical Trial.
-
Efficacy of Clotrimazole 1% Solution Compared to Econazole Nitrate 1% + Triamcinolone Acetonide 0.1% Cream in Patient with Otomycosis.Mymensingh Med J. 2021 Jul;30(3):638-643. Mymensingh Med J. 2021. PMID: 34226449 Clinical Trial.
-
Otomycosis in immunocompetent and immunocompromised patients: comparative study and literature review.Ear Nose Throat J. 2012 Mar;91(3):114-21. doi: 10.1177/014556131209100308. Ear Nose Throat J. 2012. PMID: 22430336 Review.
References
-
- Khan F, Muhammad R, Khan MR, et al. Efficacy of topical clotrimazole in treatment of otomycosis. J Ayub Med Coll Abbottabad. 2013;25(1-2):78-80. - PubMed
-
- Salvat. SVT-15652 Otic Solution for the Treatment of Otomycosis NCT03686384. U.S. National Library of Medecine. Published 2021. Accessed July 26, 2023. https://www.clinicaltrials.gov/study/NCT03686384?term=SVT-15652&cond=Oto...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources