Chlamydia trachomatis TmeA promotes pedestal-like structure formation through N-WASP and TOCA-1 interactions
- PMID: 40231845
- PMCID: PMC12108077
- DOI: 10.1128/msphere.00101-25
Chlamydia trachomatis TmeA promotes pedestal-like structure formation through N-WASP and TOCA-1 interactions
Abstract
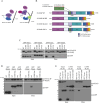
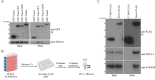
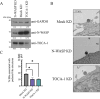
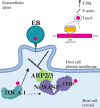
Chlamydia trachomatis (C.t.) is the causative agent of several human diseases, including the sexually transmitted infection chlamydia and the eye infection trachoma. As an obligate intracellular bacterial pathogen, invasion is critical for establishing infection and subsequent pathogenesis. During invasion, C.t. secretes effector proteins via its type III secretion system (T3SS), which manipulate host actin cytoskeletal regulation to promote bacterial entry. Previous studies identified the T3SS effector protein TmeA as a key factor in C.t. invasion, as it recruits and activates N-WASP. This interaction, in turn, activates the Arp2/3 complex, driving cytoskeletal rearrangements at the invasion site to drive C.t. uptake. In this study, we define the role of the N-WASP CRIB domain in mediating this interaction and demonstrate that TmeA functions as a mimic of Cdc42 as part of its established role in activating N-WASP. Additionally, we identified TOCA-1 as another host protein that directly interacts with TmeA. In other bacterial pathogens, notably an enterohemorrhagic E. coli, N-WASP and TOCA-1 are hijacked to mediate pedestal formation. Using siRNA-mediated knockdown of N-WASP and TOCA-1, followed by transmission electron microscopy, we found that both proteins are important for C.t.-mediated pedestal-like structure formation. Collectively, these findings expand our understanding of the intricacies of C.t. invasion, highlighting how TmeA-mediated interactions with N-WASP and TOCA-1 contribute to pedestal-like structure formation, which may represent an early step in C.t. infection.
Importance: Chlamydia trachomatis (C.t.) is an obligate intracellular bacterial pathogen that poses a significant threat to human health, being associated with various diseases, including chlamydia-the most prevalent bacterial sexually transmitted infection-and trachoma. Although often asymptomatic, chlamydia infections can lead to severe complications, such as infertility, ectopic pregnancy, and an increased risk of cervical and ovarian cancers. As an intracellular pathogen, host cell invasion is critical for C.t. survival and pathogenesis. In this study, we provide new insights into the interactions between the C.t. invasion effector protein TmeA and the host proteins N-WASP and TOCA-1, revealing that both host proteins are involved in pedestal-like structure formation during early stages of C.t. infection. These findings deepen our understanding of the mechanisms underlying TmeA-mediated host cell invasion and highlight a key pathway contributing to C.t.-mediated pathogenesis.
Keywords: Chlamydia trachomatis; N-WASP; T3SS; TOCA-1; TmeA; invasion; pedestal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Chlamydia trachomatis TmeA promotes pedestal formation through N-WASP and TOCA-1 interactions.bioRxiv [Preprint]. 2024 Oct 31:2024.10.31.621191. doi: 10.1101/2024.10.31.621191. bioRxiv. 2024. Update in: mSphere. 2025 May 27;10(5):e0010125. doi: 10.1128/msphere.00101-25. PMID: 39554107 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

