Asprosin-FABP5 Interaction Modulates Mitochondrial Fatty Acid Oxidation through PPARα Contributing to MASLD Development
- PMID: 40231957
- PMCID: PMC12140288
- DOI: 10.1002/advs.202415846
Asprosin-FABP5 Interaction Modulates Mitochondrial Fatty Acid Oxidation through PPARα Contributing to MASLD Development
Abstract
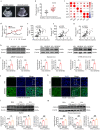
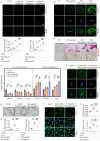
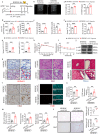
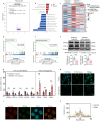
Alterations in liver metabolism play a pivotal role in the development and progression of metabolic dysfunction-associated steatotic liver disease (MASLD). Asprosin is reported to be released from white adipose tissue during fasting and targets the liver. However, the role of asprosin, especially from organs other than adipose tissue, in MASLD remains poorly understood. These findings demonstrate that plasma asprosin levels are significantly elevated in MASLD patients and animal models. Additionally, asprosin expression increased in the liver of MASLD mice. Hepatocyte-specific overexpression of asprosin impairs mitochondrial fatty acid β-oxidation (FAO), whereas its knockdown not only enhances FAO in mice but also compensates for fenofibrate's limitations in MASLD treatment. Mechanistic investigations reveal that the interaction of asprosin with FABP5 facilitates its abnormal nuclear localization, and asprosin directly bound to and inhibites peroxisome proliferator-activated receptor elements (PPREs), which negatively regulated PPARα transcriptional activity, and disrupts hepatic FAO pathways. GalNAc-siRNAs targeting hepatic FABP5 ameliorate hepatic steatosis. These findings reveal that the secretory adipose factor asprosin is expected to act as a biological marker for early clinical diagnosis and prognostic evaluation of MASLD. Moreover, targeting hepatic asprosin gene inhibition and GalNAc-siRNAs to inhibit hepatic FABP5 both offer potential therapeutic benefits in the treatment of MASLD.
Keywords: FABP5; PPARα; asprosin; fenofibrate; hepatic steatosis; insulin resistance; mitochondrial fatty acid β‐oxidation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lazarus J. V., Newsome P. N., Francque S. M., Kanwal F., Terrault N. A., Reply R. M. E., Hepatology 2024, 79, E93. - PubMed
-
- Kalligeros M., Vassilopoulos A., Vassilopoulos S., Victor D. W., Mylonakis E., Noureddin M., Clin. Gastroenterol. Hepatol. 2023, 22, 1330 - PubMed
-
- Targher G., Byrne C. D., Tilg H., Gut 2024, 73, 691. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
