Cytomegalovirus-RNA Accurately Identifies Clinically Significant Infection Needing Preemptive Therapy in Liver Transplanted Children: A Proof-of-Concept Study
- PMID: 40232168
- PMCID: PMC11998893
- DOI: 10.1002/jmv.70347
Cytomegalovirus-RNA Accurately Identifies Clinically Significant Infection Needing Preemptive Therapy in Liver Transplanted Children: A Proof-of-Concept Study
Abstract
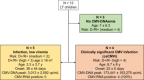
Preemptive therapy (PET) is safe and effective in controlling Cytomegalovirus (CMV) infection after pediatric liver transplantation (LT) and allows to observe the kinetics of quantitative CMV-DNA viral load till it reaches the treatment thresholds. While early detection of low-to-moderate CMV-DNA levels may not indicate active viral replication, awaiting the viral load to exceed the treatment threshold may lead to viremic breakthroughs and CMV disease. We assessed the capacity of quantitative CMV-RNA (UL21.5 mRNA) to identify active viral replication and its accuracy in identifying clinically significant CMV infection (csCMVi) needing PET in LT children. One-hundred and forty-four comparative quantitative CMV-RNA and CMV-DNA determinations were obtained from 12 children followed prospectically for 6 months after LT. Of 52 CMV-DNA-positive specimens, 17 (32%) were also CMV-RNA-positive, while CMV-RNA was undetectable in CMV-DNA-negative specimens. All children with csCMVi had early detectable CMV-RNA, peaking simultaneously to CMV-DNA (median CMV-DNA: 65 906 cp/mL; median CMV-RNA: 767 cp/mL); conversely, none of those with persistently low DNAemia proved CMV-RNA-positive. In this first pilot study, CMV-RNA had 100% sensitivity and specificity in identifying children needing PET after pediatric LT. The early detection of CMV-RNA marks significant CMV infection/reactivation, thus allowing to avoid unnecessary antiviral treatment.
Keywords: Cytomegalovirus; epidemiology; immune system; infection; latent infection; pathogenesis; transplantation; virus classification.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Figures


References
-
- Bosch W., Heckman M. G., Diehl N. N., Shalev J. A., Pungpapong S., and Hellinger W. C., “Association of Cytomegalovirus Infection and Disease With Death and Graft Loss After Liver Transplant in High‐Risk Recipients,” American Journal of Transplantation 11, no. 10 (2011): 2181–2189, 10.1111/j.1600-6143.2011.03618.x. - DOI - PubMed
-
- Knackstedt E. D., Anderson S. G., Anand R., et al., “Cytomegalovirus Prophylaxis in Pediatric Liver Transplantation: A Comparison of Strategies Across the Society of Pediatric Liver Transplantation (SPLIT) Consortium,” American Journal of Transplantation, ahead of print, October 3, 2024, 10.1016/j.ajt.2024.09.025. - DOI - PubMed
-
- Verma A., Palaniswamy K., Cremonini G., Heaton N., and Dhawan A., “Late Cytomegalovirus Infection in Children: High Incidence of Allograft Rejection and Hepatitis in Donor Negative and Seropositive Liver Transplant Recipients,” Pediatric Transplantation 21, no. 3 (2017): e12879, 10.1111/petr.12879. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

