Proliferator-Activated Receptor Alpha Inhibits Abnormal Extracellular Matrix Accumulation and Maintains Energy Metabolism in Late-Onset Fuchs Endothelial Corneal Dystrophy
- PMID: 40232711
- PMCID: PMC12007668
- DOI: 10.1167/iovs.66.4.36
Proliferator-Activated Receptor Alpha Inhibits Abnormal Extracellular Matrix Accumulation and Maintains Energy Metabolism in Late-Onset Fuchs Endothelial Corneal Dystrophy
Abstract
Purpose: Fuchs endothelial corneal dystrophy (FECD) is the most common corneal endothelial dystrophy and guttae are crucial in causing progressive loss of corneal endothelium. This study aimed to find a way to inhibit the formation of guttae in FECD.
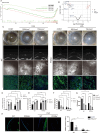
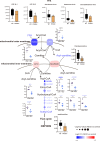
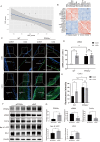
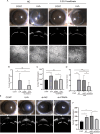
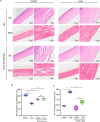
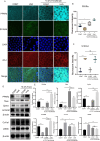
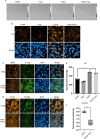
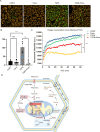
Methods and results: Mitochondria fatty acid β-oxidation (FAO) and tricarboxylic acid (TCA) cycle processes were negatively enriched in the FECD group according to gene set enrichment analysis in GSE171830. In vivo UV-A-induced late-onset FECD mouse model were established. After irradiation, aged proliferator-activated receptor alpha (PPARα-/-) mice manifested greater corneal opacity, cornea edema, and varied corneal endothelial cell morphology compared with wild-type mice. The total metabolites in cornea of aged PPARα-/- mice and wild-type mice were detected by mass spectrometry. Metabolites of the FAO pathway were decreased in corneas of PPARα-/- mice, coincident with enzymes of FAO decreased in GSE171830. The score for FAO energy metabolism was negatively related to that of the TGF-β pathway according to gene set variation analysis. The express of alpha smooth muscle actin (αSMA) and Col1a were increased in aged PPARα-/- mice and small interfering PPARα B4G12 cell lines. After irradiation, activation or overexpression of PPARα demonstrated reduced corneal endothelial damage and reversal of Descemet membrane thickening, along with downregulation of fibrosis-related genes such as αSMA and collagen type I alpha 1 (Col1a). In vitro experiments revealed that fenofibrate could reverse fibrosis and damage of cell-to-cell connections induced by TGF-β. Additionally, fenofibrate was found to alleviate mitochondrial damage in B4G12 and increase oxygen consumption rates after TGF-β treatment.
Conclusions: Overall, we suggested that the overexpression or activation of PPARα can inhibit FAO energy dysfunction of corneal endothelium and the abnormal extracellular matrix formation in Descemet's membrane, which is the primary pathology of FECD. Thus, PPARα may be a potential target for attenuating the progression of FECD.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Rapid detection of guttae area using aniline blue staining in Fuchs endothelial corneal dystrophy mouse model.Clin Exp Pharmacol Physiol. 2024 Oct;51(10):e13921. doi: 10.1111/1440-1681.13921. Clin Exp Pharmacol Physiol. 2024. PMID: 39223829
-
Comprehensive identification of dysregulated extracellular matrix molecules in the corneal endothelium of patients with Fuchs endothelial corneal dystrophy.Sci Rep. 2025 Apr 26;15(1):14654. doi: 10.1038/s41598-025-91850-y. Sci Rep. 2025. PMID: 40287463 Free PMC article.
-
Involvement of TGF-β signaling pathway-associated genes in the corneal endothelium of patients with Fuchs endothelial corneal dystrophy.Exp Eye Res. 2025 Jun;255:110334. doi: 10.1016/j.exer.2025.110334. Epub 2025 Mar 11. Exp Eye Res. 2025. PMID: 40081749
-
Mitochondrial Dysfunction and Mitophagy in Fuchs Endothelial Corneal Dystrophy.Cells. 2021 Jul 26;10(8):1888. doi: 10.3390/cells10081888. Cells. 2021. PMID: 34440658 Free PMC article. Review.
-
Transcript profile of cellular senescence-related genes in Fuchs endothelial corneal dystrophy.Exp Eye Res. 2014 Dec;129:13-7. doi: 10.1016/j.exer.2014.10.011. Epub 2014 Oct 11. Exp Eye Res. 2014. PMID: 25311168 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

