CD70 recruitment to the immunological synapse is dependent on CD20 in B cells
- PMID: 40232798
- PMCID: PMC12037035
- DOI: 10.1073/pnas.2414002122
CD70 recruitment to the immunological synapse is dependent on CD20 in B cells
Abstract
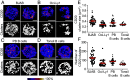
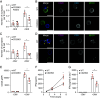
CD20 is a four-transmembrane protein expressed at the surface of B cells from late pro-B cells to memory B cells, with the exception of plasma cells. Its expression pattern makes it an attractive therapeutic target for different B cell malignancies and autoimmune diseases. Despite the clinical success of CD20-targeting antibodies, the biology of the CD20 protein is still not well understood. We investigated CD20 binding partners in the membrane of human B cells using immunoprecipitation followed by mass spectrometry analysis. We identified a molecular interaction between CD70 and CD20, and confirmed this using proximity ligation assays. CD20-CD70 spatiotemporal colocalization was validated at the plasma membrane of B cells using high-resolution microscopy. Cell surface expression of CD70 was found to be enhanced upon CD20 overexpression, suggesting a role for CD20 in stabilizing CD70 at the B cell membrane. Moreover, we observed impaired B-T cell synapse formation and defective recruitment of CD70 to the immunological synapse in the absence of CD20. Impaired synapse formation was confirmed by deleting CD20 in primary B cells, and analysis of B cells from a CD20-deficient patient. Finally, CD20-deletion resulted in diminished T cell activation and cytokine secretion. Together, this study demonstrates that CD20 interacts with CD70 at the B cell membrane, and that CD20 is required for immune synapse formation between B and T cells and consequent T cell activation.
Keywords: B lymphocytes; CD20; CD70; immune synapse.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
- ICI00023/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO)
- project 09120012010023/ZonMw (Netherlands Organisation for Health Research and Development)
- projects 12949 and 14726/KWF Kankerbestrijding (DCS)
- projects 724281 and 101112687/EC | European Research Council (ERC)
- 471011418 project A01 and SFB1160 - 256073931 project B02/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Research Materials

