Final 3-year results from the EVIDENS study, an observational study of nivolumab in non-small cell lung cancer
- PMID: 40232811
- PMCID: PMC12001549
- DOI: 10.1080/2162402X.2025.2492932
Final 3-year results from the EVIDENS study, an observational study of nivolumab in non-small cell lung cancer
Abstract
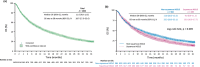
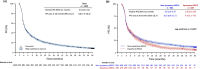
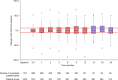
EVIDENS was a prospective, non-interventional, longitudinal study conducted in non-small cell lung cancer (NSCLC) patients receiving nivolumab in France. It recruited adults with pathologically confirmed NSCLC who initiated nivolumab between October 2016 and November 2017; the final results are reported here. Primary outcomes included baseline characteristics and 36-month overall survival (OS). Secondary outcomes included progression free survival (PFS), objective response rate (ORR), safety and health-related quality of life (HRQoL; assessed regardless of nivolumab continuation or interruption). Overall, 1423 patients were included in the analysis population (median age 66 years; non-squamous histology 69.1%; stage IV disease 91.5%; brain metastases 19.9%). Almost all patients (99.7%) had received prior chemotherapy, and most patients received nivolumab as second-line (73.5%) or later (26.1%) therapy. The 36-month OS rate was 19.7% (95% confidence interval [CI] 17.5-22.0); OS was significantly shorter in patients with squamous versus non-squamous tumors (9.8 [95% CI 8.6-11.2] months vs 11.8 [95% CI 10.2-13.2] months; p = 0.005). The 36-month PFS rate was 8.8% (95% CI 7.3-10.4). The 12-month investigator-assessed best ORR in the overall population was 20.4%. Eastern Cooperative Oncology Group performance status, smoking status, tumor histology, disease stage and liver metastasis independently predicted survival. Grade 3 and 4 treatment-related adverse events were reported in 8.0% and 0.8% of patients, respectively; eight treatment-related deaths occurred (0.005%). HRQoL was maintained with slight improvement throughout the study, without statistical significance. These results confirm that the real-world effectiveness and safety of nivolumab in these patients is similar to that observed in clinical trials.
Keywords: EVIDENS; France; nivolumab; non-small cell lung cancer; observational study.
Conflict of interest statement
FB reports institutional financial interests in AbbVie, ACEA, Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi Aventis and Takeda. DD has received institutional research grants or contracts from Roche, AstraZeneca, Lilly, Bristol Myers Squibb, Boehringer Ingelheim, Chiesi, Chugaï, Pfizer, MSD, Novartis, GSK, Sandoz, Takeda, OSE Immunotherapeutics, Bayer, Janssen, Sanofi Aventis and Amgen; has received consulting fees from Roche, Bristol Myers Squibb, Pfizer, OSE Immunotherapeutics and Amgen; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Chugaï, Roche, Novartis, Pfizer, MSD, Bristol Myers Squibb, Boehringer Ingelheim, Gilead and Amgen; has received support for attending meetings and/or travel from Roche, Boehringer Ingelheim, Novartis, Pfizer, Bristol Myers Squibb, AstraZeneca, MSD and GSK; and has participated in a Safety Monitoring Board or Advisory Board for Roche, Boehringer Ingelheim, Pfizer, MSD, Bristol Myers Squibb, Novartis, AstraZeneca and Amgen. J-BA has received consulting fees from AstraZeneca and Janssen; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi, Bristol Myers Squibb and Takeda; and has received support for attending meetings and/or travel from AstraZeneca and Sanofi. Nicolas Benoit has received consulting fees from AstraZeneca. DMB has received fees from BMS for Clinical trials, Advisory boards and travel expenses. BA has received consulting fees from Bristol Myers Squibb; and has participated in a Safety Monitoring Board or Advisory Board for Bristol Myers Squibb. CAV has received fees for advisory boards, speaker, or travel expenditures from Roche, BMS, MSD, Pfizer, Astra Zeneca, Sanofi, Janssen, Amgen, Biodena care. She serves in an advisory Role, Scientific committee for IFCT, is a Principal Investigator for GFPC, and is a Member of Board of Directors, Editorial Committee for Edimark, Lettre du cancérologue, Actualités en oncologie thoracique. She is a member of the Board of Directors, President of 3C VAR OUEST. FB and YK are employees of and hold stock or stock options in Bristol Myers Squibb. F-EC is an employee of Bristol Myers Squibb. MP has received consulting fees from Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Roche, Daiichi Sankyo, Janssen, Ipsen, Esai, GSK, Eli Lilly, Pfizer, Takeda and Novocure; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, AnHeart Therapeutics, Sanofi, Pfizer, Takeda and Janssen; has received payment for expert testimony from Bristol Myers Squibb, AstraZeneca, Roche and Janssen; has received support for attending meetings and/or travel from Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Roche, Pfizer and Takeda; and has participated in a Safety Monitoring Board or Advisory Board for Roche and Pharmamar. The remaining authors report no potential conflicts of interests.
Figures

References
-
- European Medicines Agency . Opdivo (nivolumab): summary of product characteristics. 2023. [accessed 2024 Feb 26]. https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-p....
-
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-Cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. PMID: 26028407. - DOI - PMC - PubMed
-
- Barlesi F, Dixmier A, Debieuvre D, Raspaud C, Auliac JB, Benoit N, Bombaron P, Moro-Sibilot D, Audigier-Valette C, Asselain B, et al. Effectiveness and safety of nivolumab in the treatment of lung cancer patients in France: preliminary results from the real-world EVIDENS study. Oncoimmunology. 2020;9(1):1744898. doi: 10.1080/2162402x.2020.1744898. PMID: 33457089. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
