Exploring [11C]CPPC as a CSF1R-targeted PET imaging marker for early Parkinson's disease severity
- PMID: 40232849
- PMCID: PMC12165784
- DOI: 10.1172/JCI186591
Exploring [11C]CPPC as a CSF1R-targeted PET imaging marker for early Parkinson's disease severity
Abstract
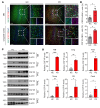
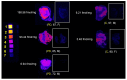
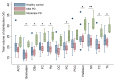
BACKGROUNDMicroglia-mediated brain immune changes play a role in the pathogenesis of Parkinson's disease (PD), but imaging microglia in living people with PD has relied on positron emission tomography (PET) ligands that lack specificity in labeling immune cells in the nervous system. We aimed to develop imaging of colony stimulating factor 1 receptor (CSF1R) as a microglial-sensitive marker of innate immunity.METHODSIHC using a CSF1R antibody evaluated colocalization with Iba-1 in PD (n = 4) and control (n = 4) human brain samples. Autoradiography using a CSF1R tritiated ligand in human brain samples from individuals with PD (n = 5) and in a control group (n = 4) was performed to obtain Bmax. PET imaging using a CSF1R radioligand was performed in 10 controls and 12 people with PD, and VT was compared between groups and correlated with disease severity.RESULTSIHC of CSF1R in human brain samples shows colocalization with Iba-1 and is significantly increased in brain samples from individuals with PD compared with individuals in a control group. Autoradiography revealed significantly increased CSF1R ligand binding in the inferior parietal cortex of patients with PD. [11C]CPPC PET showed higher binding in people with moderate PD compared with people in a control group and ligand binding correlated with more severe motor disability and poorer verbal fluency.CONCLUSIONThis study underscores the significance of CSF1R imaging as a promising biomarker for brain immune function in Parkinson's disease, which may be associated with cognitive and motor disease severity.FUNDINGPET imaging: the Michael J. Fox Foundation and the RMS Family Foundation. Radiotracer development: NIH (R01AG066464 and P41 EB024495). Postmortem brain tissues: NIH P30 AG066507 and BIOCARD study NIH U19 AG033655.
Keywords: Inflammation; Innate immunity; Neurodegeneration; Neuroscience; Parkinson disease.
Conflict of interest statement
Figures


Update of
-
Exploring [11C]CPPC as a CSF1R-targeted PET Imaging Marker for Early Parkinson's Disease Severity.medRxiv [Preprint]. 2024 Feb 13:2023.05.28.23290647. doi: 10.1101/2023.05.28.23290647. medRxiv. 2024. Update in: J Clin Invest. 2025 Apr 15;135(12):e186591. doi: 10.1172/JCI186591. PMID: 37398476 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

