Listeria monocytogenes requires phosphotransferase systems to facilitate intracellular growth and virulence
- PMID: 40233105
- PMCID: PMC12052390
- DOI: 10.1371/journal.ppat.1012492
Listeria monocytogenes requires phosphotransferase systems to facilitate intracellular growth and virulence
Abstract
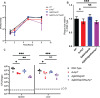
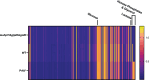
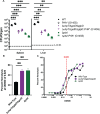
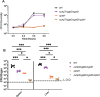
The metabolism of bacterial pathogens is exquisitely evolved to support virulence in the nutrient-limiting host. Many bacterial pathogens utilize bipartite metabolism to support intracellular growth by splitting carbon utilization between two carbon sources and dividing flux to distinct metabolic needs. For example, previous studies suggest that the professional cytosolic pathogen Listeria monocytogenes (L. monocytogenes) utilizes glycerol and hexose phosphates (e.g., Glucose-6-Phosphate) as catabolic and anabolic carbon sources in the host cytosol, respectively. However, the role of this putative bipartite metabolism in L. monocytogenes virulence has not been fully assessed. Here, we demonstrate that when L. monocytogenes is unable to consume either glycerol (ΔglpD/ΔgolD), hexose phosphates (ΔuhpT), or both (ΔglpD/ΔgolD/ΔuhpT), it is still able to grow in the host cytosol and is 10- to 100-fold attenuated in vivo suggesting that L. monocytogenes consumes alternative carbon source(s) in the host. An in vitro metabolic screen using BioLog's phenotypic microarrays unexpectedly demonstrated that WT and PrfA* (G145S) L. monocytogenes, a strain with constitutive virulence gene expression, use phosphotransferase system (PTS) mediated carbon sources. These findings contrast with the existing metabolic model that cytosolic L. monocytogenes expressing PrfA does not use PTS mediated carbon sources. We next demonstrate that two independent and universal phosphocarrier proteins (PtsI [EI] and PtsH [HPr]), essential for the function of all PTS, are critical for intracellular growth and virulence in vivo. Constitutive virulence gene expression using a PrfA* (G145S) allele in ΔglpD/ΔgolD/ΔuhpT and ΔptsI failed to rescue in vivo virulence defects suggesting phenotypes are due to metabolic disruption and not virulence gene regulation. Finally, in vivo attenuation of ΔptsI and ΔptsH was additive to ΔglpD/ΔgolD/ΔuhpT, suggesting that hexose phosphates and glycerol and PTS mediated carbon source are relevant metabolites. Taken together, these studies indicate that PTS are critical virulence factors for the cytosolic growth and virulence of L. monocytogenes.
Copyright: © 2025 Freeman et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Modulation of PrfA activity in Listeria monocytogenes upon growth in different culture media.Microbiology (Reading). 2008 Dec;154(Pt 12):3856-3876. doi: 10.1099/mic.0.2008/018283-0. Microbiology (Reading). 2008. PMID: 19047753
-
A Novel Growth-Based Selection Strategy Identifies New Constitutively Active Variants of the Major Virulence Regulator PrfA in Listeria monocytogenes.J Bacteriol. 2020 May 11;202(11):e00115-20. doi: 10.1128/JB.00115-20. Print 2020 May 11. J Bacteriol. 2020. PMID: 32179627 Free PMC article.
-
Interference of components of the phosphoenolpyruvate phosphotransferase system with the central virulence gene regulator PrfA of Listeria monocytogenes.J Bacteriol. 2007 Jan;189(2):473-90. doi: 10.1128/JB.00972-06. Epub 2006 Nov 3. J Bacteriol. 2007. PMID: 17085572 Free PMC article.
-
From hot dogs to host cells: how the bacterial pathogen Listeria monocytogenes regulates virulence gene expression.Future Microbiol. 2006 Jun;1(1):89-101. doi: 10.2217/17460913.1.1.89. Future Microbiol. 2006. PMID: 17661688 Review.
-
Listeria monocytogenes - from saprophyte to intracellular pathogen.Nat Rev Microbiol. 2009 Sep;7(9):623-8. doi: 10.1038/nrmicro2171. Epub 2009 Aug 3. Nat Rev Microbiol. 2009. PMID: 19648949 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

