Transcriptome-guided GLP-1 receptor therapy rescues metabolic and behavioral disruptions in a Bardet-Biedl syndrome mouse model
- PMID: 40233116
- PMCID: PMC12165790
- DOI: 10.1172/JCI184636
Transcriptome-guided GLP-1 receptor therapy rescues metabolic and behavioral disruptions in a Bardet-Biedl syndrome mouse model
Abstract
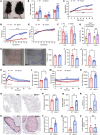
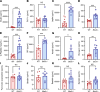
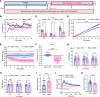
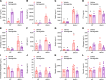
Bardet-Biedl syndrome (BBS), a ciliopathy characterized by obesity, hyperphagia, and learning deficits, arises from mutations in Bbs genes. Exacerbated symptoms occur with mutations in genes encoding the BBSome, a complex regulating primary cilia function. We investigated the mechanisms underlying BBS-induced obesity using a Bbs5-knockout (Bbs5-/-) mouse model. Bbs5-/- mice were characterized by hyperphagia, learning deficits, glucose/insulin intolerance, and disrupted metabolic hormones, phenocopying human BBS. White adipose tissue in these mice had a unique immunophenotype, with increased proinflammatory macrophages and dysfunctional Tregs, suggesting a mechanism for adiposity distinct from those of typical obesity models. Additionally, Bbs5-/- mice exhibited pancreatic islet hyperplasia but failed to normalize blood glucose, suggesting defective insulin action. Hypothalamic transcriptomics revealed dysregulation of endocrine signaling pathways, with functional analyses confirming defects in insulin, leptin, and cholecystokinin (CCK) signaling, while glucagon-like peptide-1 receptor (GLP-1R) responsiveness was preserved. Notably, treatment with a GLP-1RA effectively alleviated hyperphagia and body weight gain, improved glucose tolerance, and regulated circulating metabolic hormones in Bbs5-/- mice. This study suggests that Bbs5-/- mice represent a valuable translational model of BBS for understanding pathogenesis and developing better treatments. Our findings highlight the therapeutic potential of GLP-1RAs for managing BBS-associated metabolic dysregulation, indicating that further investigation for clinical application is warranted.
Keywords: Endocrinology; Macrophages; Metabolism; Monogenic diseases; Obesity.
Conflict of interest statement
Figures


Comment in
- Should GLP-1 receptor agonist therapy be used to treat obesity in Bardet-Biedl syndrome? doi: 10.1172/JCI191822
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

