Midbrain cytotoxic T cells as a distinct neuropathological feature of progressive supranuclear palsy
- PMID: 40233181
- PMCID: PMC12316005
- DOI: 10.1093/brain/awaf135
Midbrain cytotoxic T cells as a distinct neuropathological feature of progressive supranuclear palsy
Abstract
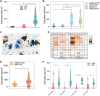
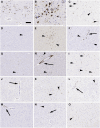
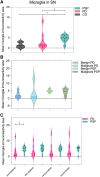
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder characterized by four-repeat (4R) tau protein deposition. The substantia nigra (SN) and midbrain tegmentum nuclei (MBT) are consistently affected. Lymphocyte infiltrates are scarce in the brains of patients with neurodegenerative diseases, although a few reports have described their presence in the α-synucleinopathy Parkinson's disease (PD). To evaluate the cytotoxic T-cell response, serial sections spanning 120 μm of the SN were immunostained consecutively for phosphorylated tau (p-tau, AT8) or α-synuclein, cytotoxic T-cell marker and microglia marker HLA-DR. Sections were analysed with stereology software in 9 patients with PSP, 10 with PD and 6 healthy controls. We semiquantitatively scored CD8-positive cells in further brain regions. CD8 lymphocyte cell counts and microglial activation in the SN were higher in PSP than PD and controls. Furthermore, T-cell/neuron contact was observed in PSP. In multivariate models, CD8 counts were not predicted by disease duration, younger age at death or the amount of p-tau pathology. The SN and midbrain tegmentum showed more CD8 cells than the cortex. A more prominent nigral cytotoxic T-cell response in PSP than PD supports the suggestion that p-tau neuropathology in PSP might have potential relationships with autoimmune mechanisms.
Keywords: T-lymphocyte; inflammation; neuropathology; progressive supranuclear palsy; tau.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
B.C. reports funding from CurePSP Foundation and Alzheimer's Association research and fellowship grants, no other personal fees. S.L.F. receives funding from the National Health and Medical Research Council, Australia outside the submitted work. C.F. was supported by an Edmond J. Safra Fellowship in Movement Disorders funded by the Michael. J. Fox Foundation for Parkinson Research. S.H.F. has received clinic support from the Edmond J, Safra Foundation for Parkinson Research, Parkinson Foundation and the Toronto Western and General Foundation; research funding from the Michael J. Fox Foundation for Parkinson Research, NIH (Dystonia Coalition); Parkinson Canada; honoraria from the International Parkinson and Movement Disorder Society; Site PI for Clinical Trials for Alexion, Biotie, Consultancy/Speaker fees from Abbvie, Bial, Ipsen, Sunovion; Paladin; and royalties from Oxford University Press. M.C.T. receives funding from NIH, Weston Brain Foundation, Tanenbaum Institute for Research in Science of Sport, Canadian Institutes of Health Research, and in-kind funding from Roche; she has served as an advisor to Eisai, Lilly and Novo Nordisk; she conducts clinical trials for Biogen, Anavex, Janssen, Novo Nordisk, Merck, Green Valley, UCB. A.E.L. has served as an advisor for AbbVie, Amylyx, Aprinoia, Biogen, BioAdvance, Biohaven, BioVie, BlueRock, BMS, Denali, Janssen, Lilly, Pharma 2B, Sun Pharma, and UCB; received honoraria from Sun Pharma, and AbbVie; received grants from Brain Canada, Canadian Institutes of Health Research, Edmond J. Safra Philanthropic Foundation, Michael J. Fox Foundation, Parkinson Foundation, and Parkinson Canada; received publishing royalties from Elsevier, Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press. G.G.K. reports personal fees from Parexel, other funding from Rossy Family Foundation, from Edmond Safra Foundation, grants from Krembil Foundation, MSA Coalition, Michael J. Fox Foundation, Parkinson Canada, NIH, Canada Foundation for Innovation, and Ontario Research Fund outside the submitted work; in addition, G.G.K. has a shared patent for 5G4 Synuclein antibody and a pending patent for Diagnostic assays for movement disorders (18/537,455), and received royalties from Wiley, Cambridge, and Elsevier publishers. The remaining authors report no competing interests.
Figures
Similar articles
-
MAPT haplotype-associated transcriptomic changes in progressive supranuclear palsy.Acta Neuropathol Commun. 2024 Aug 17;12(1):135. doi: 10.1186/s40478-024-01839-3. Acta Neuropathol Commun. 2024. PMID: 39154163 Free PMC article.
-
Cerebral Tau Deposition in Comorbid Progressive Supranuclear Palsy and Amyotrophic Lateral Sclerosis: An [18F]-Flortaucipir and 7T MRI Study.Neurodegener Dis. 2023;23(3-4):35-42. doi: 10.1159/000536614. Epub 2024 Mar 25. Neurodegener Dis. 2023. PMID: 38527450 Free PMC article.
-
Diagnostic and prognostic value of α-synuclein seed amplification assay kinetic measures in Parkinson's disease: a longitudinal cohort study.Lancet Neurol. 2025 Jul;24(7):580-590. doi: 10.1016/S1474-4422(25)00157-7. Lancet Neurol. 2025. PMID: 40541208
-
Tau PET imaging in progressive supranuclear palsy: a systematic review and meta-analysis.J Neurol. 2023 May;270(5):2451-2467. doi: 10.1007/s00415-022-11556-3. Epub 2023 Jan 12. J Neurol. 2023. PMID: 36633672
-
Effectiveness of allied health therapy in the symptomatic management of progressive supranuclear palsy: a systematic review.JBI Database System Rev Implement Rep. 2016 Jun;14(6):148-95. doi: 10.11124/JBISRIR-2016-2002352. JBI Database System Rev Implement Rep. 2016. PMID: 27532657
Cited by
-
Neuroinflammation distinguishes HLA haplotypes in progressive supranuclear palsy.medRxiv [Preprint]. 2025 Jul 21:2025.07.21.25331869. doi: 10.1101/2025.07.21.25331869. medRxiv. 2025. PMID: 40778123 Free PMC article. Preprint.
References
-
- Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol. 2001;60:647–657. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

