Symptoms and Management of Painful Progressive Swelling in Eswatini Snakebite Patients: A Prospective Observational Study
- PMID: 40233729
- PMCID: PMC12139540
- DOI: 10.4269/ajtmh.24-0671
Symptoms and Management of Painful Progressive Swelling in Eswatini Snakebite Patients: A Prospective Observational Study
Abstract
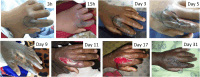
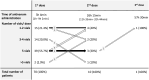
In Eswatini, bites from snakes with cytotoxic venoms inflict substantial morbidity on humans through blistering, swelling, and tissue necrosis. Despite its widespread use, there is little evidence regarding the efficacy of antivenom in preventing snakebite-induced tissue damage. We conducted a prospective observational study in nine hospitals in Eswatini to describe and quantify symptoms of local tissue toxicity. Our secondary aim was to examine the use of antivenom. Data from 125 snakebite patients with extensive or rapidly progressive swelling were analyzed. The median increase in circumference of envenomed limbs was 12%. Necrosis developed in 31 (25%) patients, primarily in distal extremities. Seventy patients (56%) received South African Institute for Medical Research (SAIMR) Polyvalent antivenom (South African Vaccine producers, Johannesburg South Africa), which was administered for indications related to local tissue damage. Upon hospital presentation, patients treated with antivenom exhibited slightly more severe swelling. Ten out of 11 patients with necrosis upon admission received antivenom. At least seven patients developed necrosis after admission despite previous antivenom therapy. In this nonrandomized observational study, no relationship was observed between the rate at which swelling receded and antivenom treatment. Adverse reactions to antivenom occurred in 49% of patients. Although our analysis has its limitations, it emphasizes the compelling need for research into the indications for and outcomes of antivenom treatment for local tissue damage.
Conflict of interest statement
Disclosures: This research was funded in whole or in part by the Wellcome Trust (grant number 217264/Z/19/Z). For the purpose of open access, the author has applied a CC BY public copyright license to any author-accepted manuscript version arising from this submission. Ethical approval was obtained from The Eswatini Health and Human Research Review Board, Eswatini (SHR204/2019), and the Research Ethics Committee of The Liverpool School of Tropical Medicine, United Kingdom (Reference LSTM REF 19-106). Informed consent from the participants was obtained before study enrollment. Permission for the participation of patients under the age of 5 years was obtained from their legal guardian. For patients aged 5–18 years, consent was sought from both the child and his or her legal guardian. In cases of emergency, or when the patient was too impaired to consent (e.g., unconsciousness, sedation, etc.), consent was deferred until the patient had sufficiently recovered. If participation was declined, all previously obtained data for research purposes were destroyed. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Figures
Similar articles
-
Antivenom for European Vipera species envenoming.Clin Toxicol (Phila). 2017 Jul;55(6):557-568. doi: 10.1080/15563650.2017.1300261. Epub 2017 Mar 28. Clin Toxicol (Phila). 2017. PMID: 28349771
-
Systematic review and meta-analysis on the efficacy of Indian polyvalent antivenom against the Indian snakes of clinical significance.Arch Toxicol. 2024 Feb;98(2):375-393. doi: 10.1007/s00204-023-03643-9. Epub 2023 Dec 28. Arch Toxicol. 2024. PMID: 38153416
-
Effect of pre-medication on early adverse reactions following antivenom use in snakebite: a systematic review and meta-analysis.Drug Saf. 2011 Oct 1;34(10):869-80. doi: 10.2165/11592050-000000000-00000. Drug Saf. 2011. PMID: 21879781
-
The Cost of Antivenom: A Cost Minimization Study using the North American Snakebite Registry.J Med Toxicol. 2025 Jul;21(3):320-326. doi: 10.1007/s13181-025-01072-x. Epub 2025 Apr 14. J Med Toxicol. 2025. PMID: 40227519 Free PMC article.
-
Regional Differences in Systemic Toxicity Following Texas Coral Snake (Micrurus tener) Envenomations.Wilderness Environ Med. 2025 Sep;36(3):328-334. doi: 10.1177/10806032251327124. Epub 2025 Mar 19. Wilderness Environ Med. 2025. PMID: 40105538
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

