Implementation of an electronic medication management support system in hospitalised polypharmacy patients: study protocol of a stepped-wedge cluster-randomised controlled trial (TOP study)
- PMID: 40233949
- PMCID: PMC12001353
- DOI: 10.1136/bmjopen-2024-084696
Implementation of an electronic medication management support system in hospitalised polypharmacy patients: study protocol of a stepped-wedge cluster-randomised controlled trial (TOP study)
Abstract
Introduction: Polypharmacy is associated with an increased risk of adverse patient outcomes across various settings, including inpatient care. To enhance the appropriateness of medication therapy management for patients during hospital stays, computerised interventions have shown promise with regard to patient safety. This study assesses whether the implementation of a clinical decision support system will optimise the process of inpatient medication therapy to prevent inappropriate medication use and thus promote patient safety.
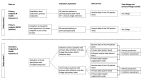
Methods and analysis: The intervention will be evaluated in a prospective, cluster-randomised controlled trial using a stepped-wedge design. The study will be conducted in 12 hospitals across Germany over a total period of 33 months. Patients will be treated according to the group status of the hospital and receive either standard care or the Transsektorale Optimierung der Patientensicherheit or trans-sectoral optimisation of patient safety intervention. The primary outcome is the combined endpoint of all-cause mortality and all-cause hospitalisation. Secondary endpoints are, for example, inappropriate prescriptions, utilisation of different health services, cost-effectiveness, as well as patient-reported outcome measures. Parameters describing the attitudes of patients and healthcare professionals towards the intervention and organisational change processes will be collected as part of the process evaluation. The primary endpoint will be evaluated using hospital and outpatient claims data from participating statutory health insurances at the population level. There are multiple secondary endpoints with data linkage of primary and secondary data at study participant level. Statistical analysis will make use of (generalised) linear mixed models or generalised estimating equations, taking account of independent covariables. All data analyses of the process evaluation will be descriptive and explorative.
Ethics and dissemination: Data collection, storage and evaluation meet all applicable data protection regulations. The trial has been approved by the Ethics Committees of the University of Wuppertal and the Medical Association of Saarland, Germany. Results will be disseminated through workshops, peer-reviewed publications and local and international conferences.
Trial registration number: DRKS00025485.
Keywords: Clinical Decision-Making; Health & safety; Hospitals; Medication Review; Polypharmacy; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SM, DL, PI, SS, CK, AD, KCR, IM, WG and JK-N report grants from the German Federal Joint Committee during the conduct of the study. DG reports grants from BARMER during the conduct of the study and a family member of DG works for and holds shares of IT company involved in the project. SG works for and holds shares of IT company involved in the project. All other authors have no competing interest to declare.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
