Four cycles of docetaxel plus cisplatin as neoadjuvant chemotherapy followed by concurrent chemoradiotherapy in stage N2-3 nasopharyngeal carcinoma: phase 3 multicentre randomised controlled trial
- PMID: 40233976
- PMCID: PMC11997745
- DOI: 10.1136/bmj-2024-081557
Four cycles of docetaxel plus cisplatin as neoadjuvant chemotherapy followed by concurrent chemoradiotherapy in stage N2-3 nasopharyngeal carcinoma: phase 3 multicentre randomised controlled trial
Abstract
Objective: To compare the effects of four cycles of docetaxel with cisplatin as a neoadjuvant chemotherapy followed by concurrent chemoradiotherapy with concurrent chemoradiotherapy alone by assessing reductions in distant metastasis and improvements in survival in patients with stage N2-3nasopharyngeal carcinoma.
Design: Phase 3, multicentre, randomised controlled trial.
Setting: Six sites in China from 23 February 2016 to 18 February 2019.
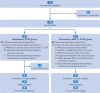
Participants: 186 participants aged ≤70 years with a diagnosis of untreated stage T1-4N2-3M0 nasopharyngeal carcinoma.
Intervention: Participants were prospectively enrolled and randomly allocated to either the neoadjuvant chemotherapy plus concurrent chemoradiotherapy group (four cycles of neoadjuvant chemotherapy (docetaxel 75 mg/m2 on day 1 and cisplatin 37.5 mg/m2 on days 2-3, every 3 weeks) followed by concurrent chemoradiotherapy (intensity modulated radiotherapy plus weekly cisplatin 40 mg/m2) or the concurrent chemoradiotherapy only group, in a 1:1 ratio.
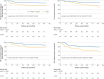
Main outcome measures: Five year distant metastasis-free survival and overall survival were analysed using the intention-to-treat approach.
Results: 93 participants were assigned to each of the neoadjuvant chemotherapy plus concurrent chemoradiotherapy and concurrent chemoradiotherapy only groups. After a median follow-up time of 76.9 (interquartile range 65.4-85.9) months, the neoadjuvant chemotherapy plus concurrent chemoradiotherapy group had superior five year distant metastasis-free survival (91.3% (95% confidence interval (CI) 85.4% to 97.2%) versus 78.2% (69.8% to 86.6%); hazard ratio 0.41 (95% CI 0.19 to 0.87); P=0.02) and five year overall survival (90.3% (84.2% to 96.4%) versus 82.6% (75.0% to 90.2%); hazard ratio 0.38 (0.18 to 0.82); P=0.01). Grade 3/4 acute toxicities were observed in 60 (65%) and 46 (51%) patients in the neoadjuvant chemotherapy plus concurrent chemoradiotherapy and concurrent chemoradiotherapy only groups, respectively (P=0.05). The higher acute toxicity observed in the neoadjuvant chemotherapy plus concurrent chemoradiotherapy group was primarily due to grade 3/4 neutropenia (43 (47%) v 10 (11%); P<0.001). No significant difference in any late toxicity was observed between the two groups, and participants in the neoadjuvant chemotherapy plus concurrent chemoradiotherapy group tended to have a better quality of life five years after enrolment.
Conclusions: Four cycles of docetaxel plus cisplatin neoadjuvant chemotherapy with concurrent chemoradiotherapy can effectively reduce distant metastasis and improve survival for patients with stage N2-3 nasopharyngeal carcinoma with manageable toxicities.
Trial registration: ClinicalTrials.gov NCT02512315.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/coi_disclosure.pdf and declare: support from the Science and Technology Planning Project of Guangdong Province, China for the submitted work; no financial relationships with any organisation that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- OuYang PY, Xiao Y, You KY, et al. Validation and comparison of the 7th and 8th edition of AJCC staging systems for non-metastatic nasopharyngeal carcinoma, and proposed staging systems from Hong Kong, Guangzhou, and Guangxi. Oral Oncol 2017;72:65-72. 10.1016/j.oraloncology.2017.07.011 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical