Magnesium-L-threonate Ameliorates Cognitive Deficit by Attenuating Adult Hippocampal Neurogenesis Impairment in a Mouse Model of Alzheimer's Disease
- PMID: 40234095
- PMCID: PMC12069926
- DOI: 10.5607/en24030
Magnesium-L-threonate Ameliorates Cognitive Deficit by Attenuating Adult Hippocampal Neurogenesis Impairment in a Mouse Model of Alzheimer's Disease
Abstract
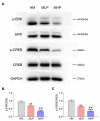
Impaired adult hippocampal neurogenesis is a key pathological mechanism contributing to memory deficits in Alzheimer's disease (AD). Recent studies have shown that elevating magnesium levels promotes neurogenesis by enhancing the neuronal differentiation of adult neural progenitor cells in vitro. Therefore, this in vivo study aims to determine if magnesium-L-threonate (MgT) can ameliorate cognitive deficit of AD mice by attenuating adult hippocampal neurogenesis impairment and to reveal the underlying mechanisms. APPswe/PS1dE9 mice were treated with different doses of MgT and ERK inhibitor PD0325901. The memory ability of each mouse was recorded by Morris Water Maze test. After cognitive test, hippocampus tissues were collected to measure the proportion of BrdU/doublecortin double-labeled cells using the flow cytometry test and assess the expression of doublecortin using PCR and Western blot. Furthermore, the activations of CREB, ERK, P38 and JNK were measured by Western blot to identify the involved mechanisms. The cognitive test confirmed that MgT treatment attenuated the memory impairment of APPswe/PS1dE9 mice. Flow cytometry test showed that Brdu/doublecortin labeled newborn neurons gradually increased following MgT administration. In line with the flow cytometry results, Western blot and PCR confirmed that MgT administration significantly increased doublecortin expression levels. Furthermore, the ratios of p-ERK/ERK and p-CREB/CREB increased with MgT elevation. In addition, these effects of MgT treatment were markedly reversed by PD0325901 supplementation. In conclusion, MgT treatment improved cognitive decline by ameliorating adult hippocampal neurogenesis impairment in this AD model, possibly via ERK/CREB activation.
Keywords: Alzheimer’s disease; Cognitive decline; Magnesium; Neurogenesis.
Figures





Similar articles
-
Magnesium-L-threonate exhibited a neuroprotective effect against oxidative stress damage in HT22 cells and Alzheimer's disease mouse model.World J Psychiatry. 2022 Mar 19;12(3):410-424. doi: 10.5498/wjp.v12.i3.410. eCollection 2022 Mar 19. World J Psychiatry. 2022. PMID: 35433327 Free PMC article.
-
Elevation of brain magnesium prevents and reverses cognitive deficits and synaptic loss in Alzheimer's disease mouse model.J Neurosci. 2013 May 8;33(19):8423-41. doi: 10.1523/JNEUROSCI.4610-12.2013. J Neurosci. 2013. Retraction in: J Neurosci. 2014 Apr 16;34(16):5733. doi: 10.1523/JNEUROSCI.1265-14.2014. PMID: 23658180 Free PMC article. Retracted.
-
Elevation of brain magnesium prevents synaptic loss and reverses cognitive deficits in Alzheimer's disease mouse model.Mol Brain. 2014 Sep 13;7:65. doi: 10.1186/s13041-014-0065-y. Mol Brain. 2014. PMID: 25213836 Free PMC article.
-
Does Impairment of Adult Neurogenesis Contribute to Pathophysiology of Alzheimer's Disease? A Still Open Question.Front Mol Neurosci. 2021 Jan 22;13:578211. doi: 10.3389/fnmol.2020.578211. eCollection 2020. Front Mol Neurosci. 2021. PMID: 33551741 Free PMC article. Review.
-
The relationship between adult hippocampal neurogenesis and cognitive impairment in Alzheimer's disease.Alzheimers Dement. 2024 Oct;20(10):7369-7383. doi: 10.1002/alz.14179. Epub 2024 Aug 21. Alzheimers Dement. 2024. PMID: 39166771 Free PMC article. Review.
Cited by
-
The Role of Magnesium in Depression, Migraine, Alzheimer's Disease, and Cognitive Health: A Comprehensive Review.Nutrients. 2025 Jul 4;17(13):2216. doi: 10.3390/nu17132216. Nutrients. 2025. PMID: 40647320 Free PMC article. Review.
References
-
- Kaji S, Berghoff SA, Spieth L, Schlaphoff L, Sasmita AO, Vitale S, Büschgens L, Kedia S, Zirngibl M, Nazarenko T, Damkou A, Hosang L, Depp C, Kamp F, Scholz P, Ewers D, Giera M, Ischebeck T, Wurst W, Wefers B, Schifferer M, Willem M, Nave KA, Haass C, Arzberger T, Jäkel S, Wirths O, Saher G, Simons M. Apolipoprotein E aggregation in microglia initiates Alzheimer's disease pathology by seeding β-amyloidosis. Immunity. 2024;57:2651–2668.e12. doi: 10.1016/j.immuni.2024.09.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

