Baicalin restores innate lymphoid immune imbalance during exacerbation of COPD
- PMID: 40234295
- PMCID: PMC12000166
- DOI: 10.1007/s12026-025-09629-2
Baicalin restores innate lymphoid immune imbalance during exacerbation of COPD
Abstract
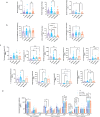
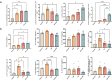
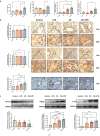
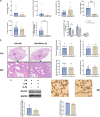
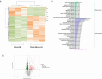
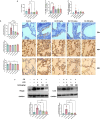
Chronic obstructive pulmonary disease (COPD) is characterized by immune dysregulation, including altered innate lymphoid cell (ILC) immune responses, particularly during exacerbations (ECOPD). Baicalin, a natural compound prevalent in various herbal medicines, has shown promise as a therapeutic candidate in ECOPD. However, its potential and molecular mechanism for addressing ILC immune imbalance during ECOPD remain poorly understood. First, this study conducted a cross-sectional analysis of ILC immune responses in stable COPD patients and those experiencing exacerbations. Then, clinical findings of skewed ILC immunity were validated in cigarette smoke and lipopolysaccharide-induced ECOPD mouse models. Lastly, the therapeutic effect of baicalin on restoring ILC immune homeostasis was investigated in experimental ECOPD mouse models. Significant downregulation of ILC2 immunity was observed during COPD exacerbations, accompanied by increased ILC1 and ILC3 responses, particularly in cases associated with bacterial infections. Notably, elevated IL-22 levels were observed in this group. Administration of recombinant IL-22 in ECOPD mouse models disrupted lung ILC homeostasis, specifically inhibiting the accumulation of ILC2. Proteomics and transcriptomics analyses suggested IL-22 as a mediator of type 2 immune suppression by creating a molecular environment that favors type 1 and type 3 immunity. Treatment with baicalin effectively restored ILC2 immunity by enhancing the recruitment and activation of lung ILC2 while suppressing ILC1 and ILC3 responses. Importantly, baicalin attenuated IL-22 production from lung ILC3, highlighting its potential as an IL-22 inhibitor. Baicalin demonstrates potential as a therapeutic strategy for addressing ILC immune imbalance in COPD exacerbations, particularly by restoring ILC2 immunity and partially inhibiting IL-22 production. Clinical registration The cross-sectional study was registered with the Chinese Clinical Trial Registry (ChiCTR2100050683).
Keywords: Baicalin; Exacerbation of chronic obstructive pulmonary disease; Immune imbalance; Innate lymphoid cells; Interleukin-22.
© 2025. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: The authors declare that they have no conflict of interest.
Figures





Similar articles
-
Elastin-derived peptides favor type 2 innate lymphoid cells in chronic obstructive pulmonary disease.Am J Physiol Lung Cell Mol Physiol. 2024 Jun 1;326(6):L812-L820. doi: 10.1152/ajplung.00306.2023. Epub 2024 May 7. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38712445
-
Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs.Nat Immunol. 2016 Jun;17(6):626-35. doi: 10.1038/ni.3443. Epub 2016 Apr 25. Nat Immunol. 2016. PMID: 27111143 Free PMC article.
-
Qingke Pingchuan granules alleviate airway inflammation in COPD exacerbation by inhibiting neutrophil extracellular traps in mice.Phytomedicine. 2025 Jan;136:156283. doi: 10.1016/j.phymed.2024.156283. Epub 2024 Nov 23. Phytomedicine. 2025. PMID: 39616733
-
The Role of Innate Lymphoid Cells in Chronic Respiratory Diseases.Front Immunol. 2021 Sep 22;12:733324. doi: 10.3389/fimmu.2021.733324. eCollection 2021. Front Immunol. 2021. PMID: 34630416 Free PMC article. Review.
-
Role of Innate Lymphoid Cells in Lung Disease.Iran J Allergy Asthma Immunol. 2015 Aug;14(4):346-60. Iran J Allergy Asthma Immunol. 2015. PMID: 26547702 Review.
References
MeSH terms
Substances
Grants and funding
- 2021ZD01/Sichuan Provincial Administration of Traditional Chinese Medicine
- 2023MS519/Sichuan Provincial Administration of Traditional Chinese Medicine
- 81900039/National Natural Science Foundation of China
- 82474368/National Natural Science Foundation of China
- 2023YFH0072/Science & Technology Department of Sichuan Province
LinkOut - more resources
Full Text Sources
Medical

