ATM knock out alters calcium signalling and augments contraction in skeletal muscle cells differentiated from human urine-derived stem cells
- PMID: 40234386
- PMCID: PMC12000312
- DOI: 10.1038/s41420-025-02485-x
ATM knock out alters calcium signalling and augments contraction in skeletal muscle cells differentiated from human urine-derived stem cells
Abstract
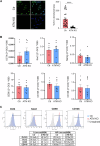
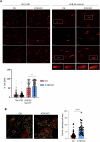
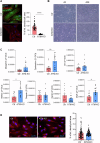
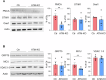
Ataxia-telangiectasia (A-T) is a rare neurodegenerative disorder caused by the deficiency of the serine/threonine kinase ataxia telangiectasia mutated (ATM) protein, whose loss of function leads to altered cell cycle, apoptosis, oxidative stress balance and DNA repair after damage. The clinical manifestations are multisystemic, among them cerebellar degeneration and muscular ataxia. The molecular mechanism by which ATM loss leads to A-T is still uncertain and, currently only symptomatic treatments are available. In this study, we generated a functional skeletal muscle cell model that recapitulates A-T and highlights the role of ATM in calcium signalling and muscle contraction. To this aim, by using CRISPR/Cas9 technology, we knocked out the ATM protein in urine-derived stem cells (USCs) from healthy donors. The resulting USCs-ATM-KO maintained stemness but showed G2/S cell cycle progression and an inability to repair DNA after UV damage. Moreover, they showed increased cytosolic calcium release after ATP stimulation to the detriment of the mitochondria. The alterations of calcium homoeostasis were maintained after differentiation of USCs-ATM-KO into skeletal muscle cells (USC-SkMCs) and correlated with impaired cell contraction. Indeed, USC-SkMCs-ATM-KO contraction kinetics were dramatically accelerated compared to control cells. These results highlight the relevant function of ATM in skeletal muscle, which is not only dependent on a non-functional neuronal communication, paving the way for future studies on a muscular interpretation of A-T ataxia.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Lee JH, Paull TT. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat Rev Mol Cell Biol. 2021;22:796–814. - PubMed
-
- Amirifar P, Ranjouri MR, Lavin M, Abolhassani H, Yazdani R, Aghamohammadi A. Ataxia-telangiectasia: epidemiology, pathogenesis, clinical phenotype, diagnosis, prognosis and management. Expert Rev Clin Immunol. 2020;16:859–71. - PubMed
-
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–69. - PubMed
-
- McKinnon PJ. ATM and the molecular pathogenesis of Ataxia Telangiectasia. Annu Rev Pathol Mech Dis. 2012;7:303–21. - PubMed
-
- Paull TT, Lee JH. The Mre11/Rad50/Nbs1 complex and its role as a DNA double-strand break sensor for ATM. Cell Cycle. 2005;4:737–40. - PubMed
Grants and funding
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- C13C22000590006 - ID T4-AN-04/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

