FTO controls CD8+ T cell survival and effector response by modulating m6A methylation of Fas
- PMID: 40234389
- PMCID: PMC12000336
- DOI: 10.1038/s41419-025-07606-z
FTO controls CD8+ T cell survival and effector response by modulating m6A methylation of Fas
Abstract
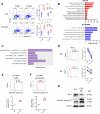
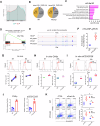
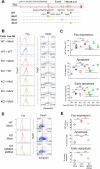
Functional CD8+ T cell immunity is essential for immune surveillance and host defense against infection and tumors. Epigenetic mechanisms, particularly RNA modification, in controlling CD8+ T cell immune response is not fully elucidated. Here, by T cell-specific deletion of fat mass and obesity-associated protein (FTO), a critical N6-methyladenosine (m6A) demethylase, we revealed that FTO was indispensable for adequate CD8+ T cell immune response and protective function. FTO ablation led to considerable cell death in activated CD8+ T cells, which was attributed to cell apoptosis. MeRIP-seq analysis revealed an increase in m6A methylation on Fas mRNA in FTO-deficient CD8+ T cells. The loss of FTO promoted Fas expression via enhancing the Fas mRNA stability, which depended on the m6A reader insulin-like growth factor-2 mRNA-biding proteins 3 (IGF2BP3). Mutation of the Fas m6A sites or knockdown IGF2BP3 could normalize the upregulated Fas expression and apoptosis levels caused by FTO ablation in CD8+ T cells. Our findings delineate a novel epigenetic regulatory mechanism of FTO-mediated m6A modification in supporting CD8+ T cell survival and effector responses, providing new insights into understanding the post-transcriptional regulation in CD8+ T cell immunological functions and the potential therapeutic intervention.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods used in this study were performed in accordance with the relevant guidelines and regulations. This study does not include human participants or human materials; therefore, obtaining informed consent for participation is not applicable. The animals used in this study were treated in accordance with the Institutional Animal Care and Use Committee of Xi’an Jiaotong University, Xi’an Center for Disease Control, and Suzhou Institute of Systems Medicine (XJTUAE2022-1160, XJTUAE2022-1161).
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

