Real-time functional proteomics enhances therapeutic targeting in precision oncology molecular tumor boards
- PMID: 40234655
- PMCID: PMC12000509
- DOI: 10.1038/s41698-025-00868-y
Real-time functional proteomics enhances therapeutic targeting in precision oncology molecular tumor boards
Abstract
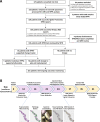
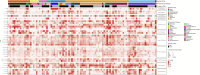
Collaborative review of molecular profiling data by multidisciplinary molecular tumor boards (MTB) is increasingly important for improving patient management and outcomes, though currently relies nearly exclusively on nucleic acid next-generation sequencing (NGS) and limited panels of immunohistochemistry-based protein abundance data. We examined the feasibility of incorporating real-time laser microdissection (LMD) enrichment of tumor epithelium and commercial CLIA-based reverse phase protein array (RPPA) protein drug target expression/activation profiling into our cancer center's MTB to complement standard clinical NGS-based profiling. The LMD-RPPA workflow was performed within a therapeutically permissive timeframe with a median dwell time of nine days, during which specimens were processed outside of standard clinical workflows. The RPPA-generated data supported additional and/or alternative therapeutic considerations for 54% of profiled patients following review by the MTB. These findings suggest that integrating proteomic/phosphoproteomic and NGS-based genomic data creates opportunities to further personalize clinical decision-making for precision oncology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: T.P.C. is a ThermoFisher Scientific, Inc. SAB member and receives research funding from AbbVie. E.F.P. is a SAB member and consultant to Ignite Proteomics, Inc., and receives research funding from Genentech, Pfizer, Mirati, Springworks Therapeutics, Deciphera, AbbVie, and is a co-inventor of the RPPA Technology described herein, and related HER2 biomarker patents and receives royalties on the related license agreements. K.N. is an independent consultant and receives funding from Catalyst Medical Media, Clinical Care Options, Global Healthcare Marketing & Communications, Health and Wellness Partners, Interactive Forums, MphaR, and PharMecha. J.D., B.C., and C.M. are full-time employees of Ignite Proteomics, Inc.
Figures
References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA: Cancer J. Clin.74, 12–49 (2024). - PubMed
-
- Goldenberg, M. M. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin. Ther.21, 309–318 (1999). - PubMed
-
- Hertel, L. W. et al. Evaluation of the antitumor activity of gemcitabine (2’,2’-difluoro-2’-deoxycytidine). Cancer Res.50, 4417–4422 (1990). - PubMed
LinkOut - more resources
Full Text Sources

