Overcoming extracellular vesicle-mediated fratricide improves CAR T cell treatment against solid tumors
- PMID: 40234680
- PMCID: PMC12303731
- DOI: 10.1038/s43018-025-00949-8
Overcoming extracellular vesicle-mediated fratricide improves CAR T cell treatment against solid tumors
Abstract
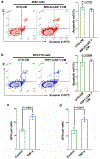
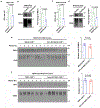
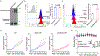
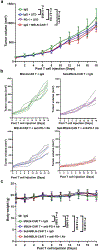
The efficacy of chimeric antigen receptor (CAR) T cells against solid tumors is limited. The molecular mechanisms underlying CAR T cell resistance are yet to be elucidated and new strategies need to be developed to improve treatment outcomes. Here we report that solid tumors respond to CAR T cells by upregulating the secretion of small extracellular vesicles carrying tumor antigens, which are horizontally transferred to CAR T cells, leading to antigen recognition and CAR T cell fratricide. Engineered CAR T cells armored with Serpin B9, a major granzyme B inhibitor, show decreased fratricide and increased vitality, tumor infiltration, and antitumor activity in female mice. Moreover, Serpin B9-armored CAR T cells show higher efficacy than parental CAR T cells in treating solid tumors when combined with the anti-programmed death 1 antibody. Our study demonstrates a mechanism that limits CAR T cell function and suggests an improved strategy in tumor treatment.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures












References
-
- Albelda SM CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat Rev Clin Oncol 21, 47–66 (2024). - PubMed
-
- Hou AJ, Chen LC & Chen YY Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov 20, 531–550 (2021). - PubMed
-
- Labanieh L & Mackall CL CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

